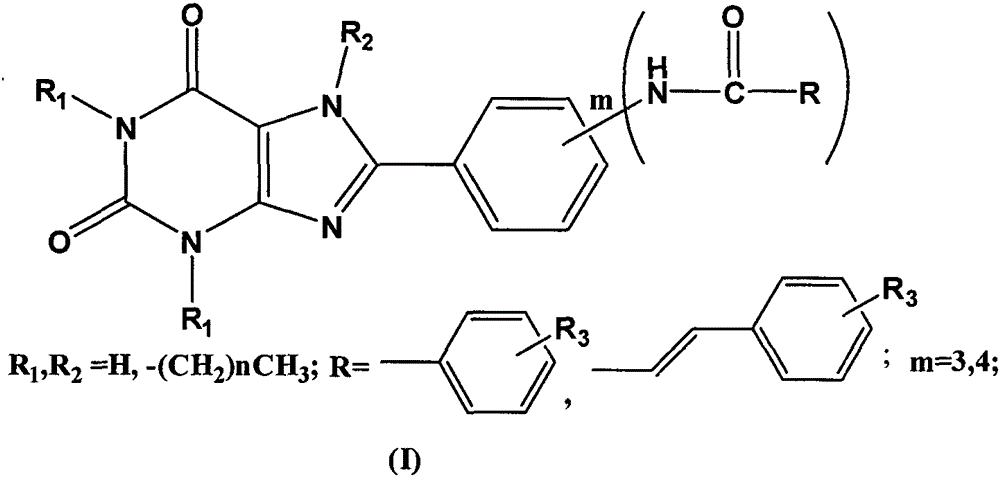
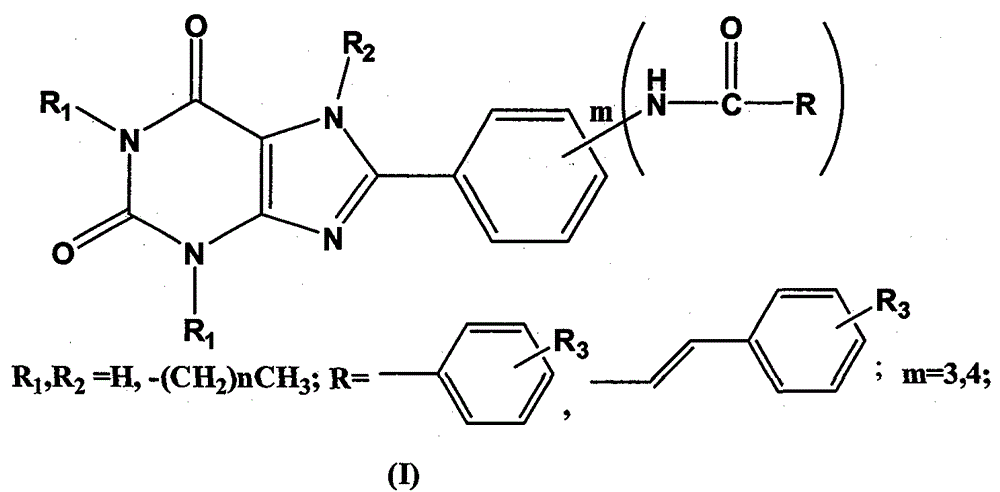
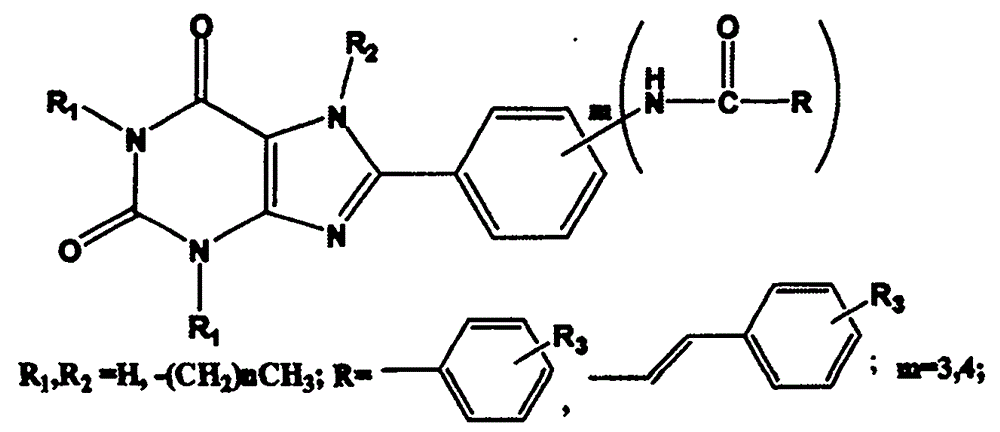
Preparation and application of 8-phenylxanthine derivatives
A technology of phenylxanthine and its derivatives, which is applied in the field of preparation and anti-tumor activity, can solve the problems that the anti-tumor activity has not been reported in the literature, and achieve the effects of reasonable design of synthesis reaction, high yield and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of 8-[3-(benzamido) phenyl]-1,3,-dimethylxanthine
[0033] First prepare 3-benzamide benzoic acid. Dissolve m-aminobenzoic acid (3.00g) in dichloromethane (40ml), add triethylamine dropwise until the solution is clear, continue stirring for 30min, slowly add benzoyl chloride dropwise, until a large amount of white precipitates are formed, continue stirring for 2h, until After the reaction is complete, filter with suction, wash with water, dry, and recrystallize with acetic acid to obtain white crystals, filter with suction, and dry.
[0034] Get the 3-benzamide benzoic acid (2.2mmol) prepared above and 5,6-diamino-1,3-dimethyluracil (2.2mmol), EDC.HCl (2.21mol) and dioxane ( 30ml) and water (30ml) were mixed evenly, and after stirring for 30min, it turned yellow and turbid, and continued to stir for 8h until the reaction was complete; put it in the refrigerator to cool, filter, and wash with water to obtain a white solid; dissolve the whit...
Embodiment 2
[0035] Example 2: Preparation of 8-[3-(benzamido)phenyl]-1,3,7-trimethylxanthine
[0036] Take the 8-[3-(benzamido)phenyl]-1,3-dimethylxanthine (1.0mmol) prepared above and anhydrous K 2 CO 3 (1.0mmol) were mixed, DMF (20ml) was added, CH was added dropwise 3 I (1.0 mmol), refluxed at 60°C for 1 h, removed the insoluble matter by suction filtration, and obtained a clear light yellow liquid, added an appropriate amount of water, precipitated a white precipitate, washed with hot methanol, and recrystallized with DMSO to obtain the product with a yield of 40%. The parameters of the product are as follows: m.p.>300°C; ESI-MS: m / z=390[M+H] + ; 1 H-NMR (CDCl 3 )δ: 3.41(s, 3H), 3.60(s, 3H), 4.10(s, 3H), 7.46(d, 2H, J=8Hz), 7.49(t, 1H, J=8Hz), 7.55(d, 1H, J=12Hz), 8.7.70(d, 2H, J=12Hz), 7.87(d, 2H, J=12Hz), 8.03(s, 1H), 10.520(s, 1H).
Embodiment 3
[0037]Example 3: Preparation of 8-[4-(4-methyl-benzamido)phenyl]-1,3-dimethylxanthine
[0038] 4-(4-Methoxy)benzamide benzoic acid is first prepared. According to the method for preparing 3-benzamide benzoic acid among the embodiment 1, wherein just replace benzoyl chloride with 4-methoxybenzoyl chloride, replace 3-aminobenzoic acid with p-aminobenzoic acid.
[0039] Take the 4-(4-methoxy)-benzoylbenzoic acid (2.2mmol) prepared above and 5,6-diamino-1,3-dimethyluracil (2.2mmol), EDC.HCl (2.21 mol) and the mixed solution of dioxane (30ml) and water (30ml) were mixed evenly, and after stirring for 30min, it became yellow and turbid, and continued to stir for 8h until the reaction was complete; cool in the refrigerator, filter, and wash with water to obtain a white solid; Dissolve the white solid in a mixed solvent of 1NNaOH (10ml) and dioxane (10ml), heat to turn into a brown clear liquid, heat at 90°C-105°C for 8 hours, until complete reaction, cool to 0°C, add dilute hydrochl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com