Compound amino acid injection (18AA-IV) composition
A compound amino acid, injection technology, applied in drug combination, metabolic diseases, peptide/protein components, etc., can solve problems such as harm to patients, affecting product qualification rate, unqualified antihypertensive substances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
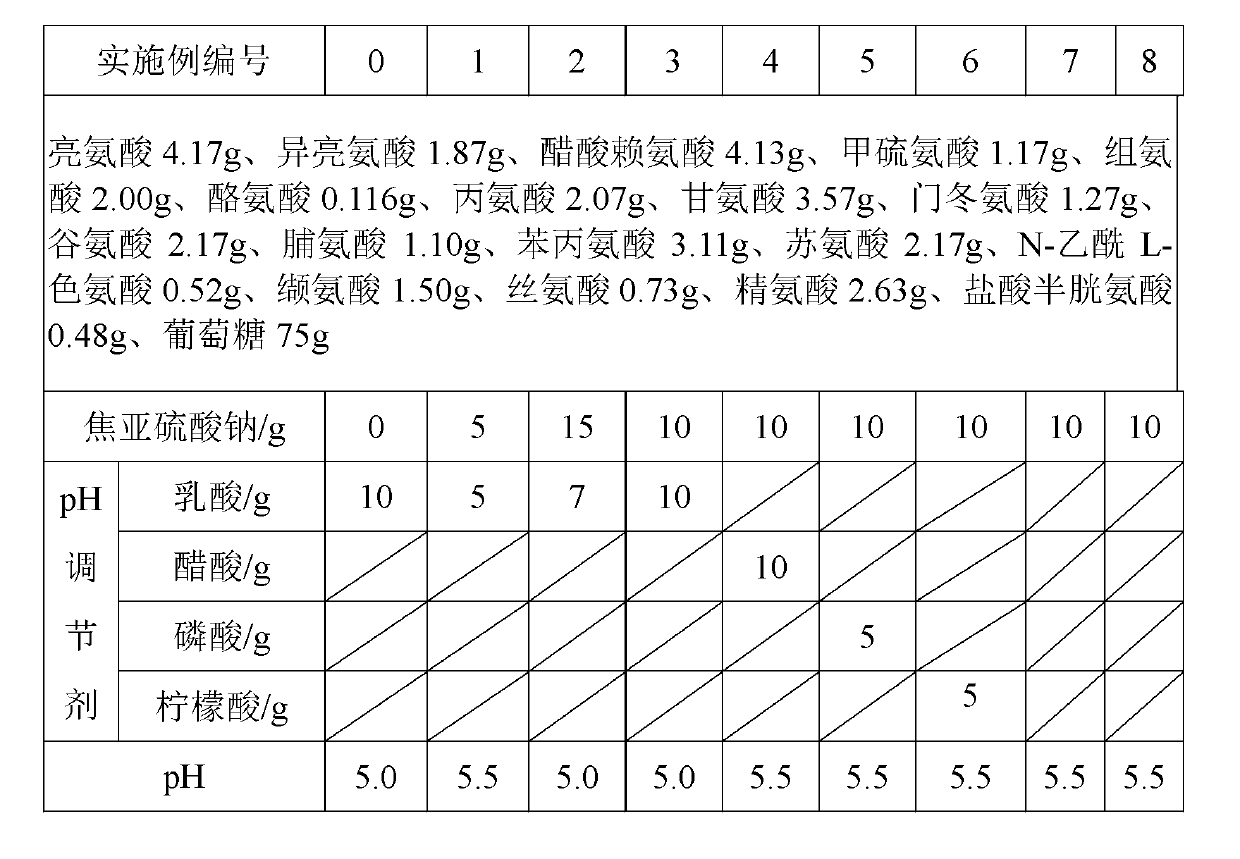
[0015] In the embodiment, the prescription in the following table is the feed amount calculated by 10000ml, and the subpackage adopts 250ml glass bottle. Sterilization was performed by autoclaving at 105°C for 45 minutes. After adding the pH regulator, adjust the pH to 5.0-5.5 with 0.1M sodium hydroxide solution or hydrochloric acid. (If the pH is qualified, you don’t need to add more)
[0016] Described injection adopts following method to prepare
[0017] 1) Take 15-25% of the prescribed amount of water for injection, put in the prescribed amount of glucose to dissolve, add 0.2% (g / ml) medicinal charcoal, stir and place for 30 minutes, and set aside.
[0018] 2) Take 35% to 45% of the prescribed amount of water for injection, under the protection of nitrogen, heat to 40~60°C, add arginine, tyrosine, isoleucine, valine, proline, leucine Acid, Lysine Acetate, Methionine, Threonine, Alanine, Glutamic Acid, Aspartic Acid, Serine, N-Acetyl-L-Tryptophan, Glycine, Phenylalanine,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com