Compound amino acid injection (15HBC) composition
A technology for injections and valine, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations with non-active ingredients, etc. It can solve problems such as unqualified antihypertensive substances, affecting product qualification rate, and harm to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
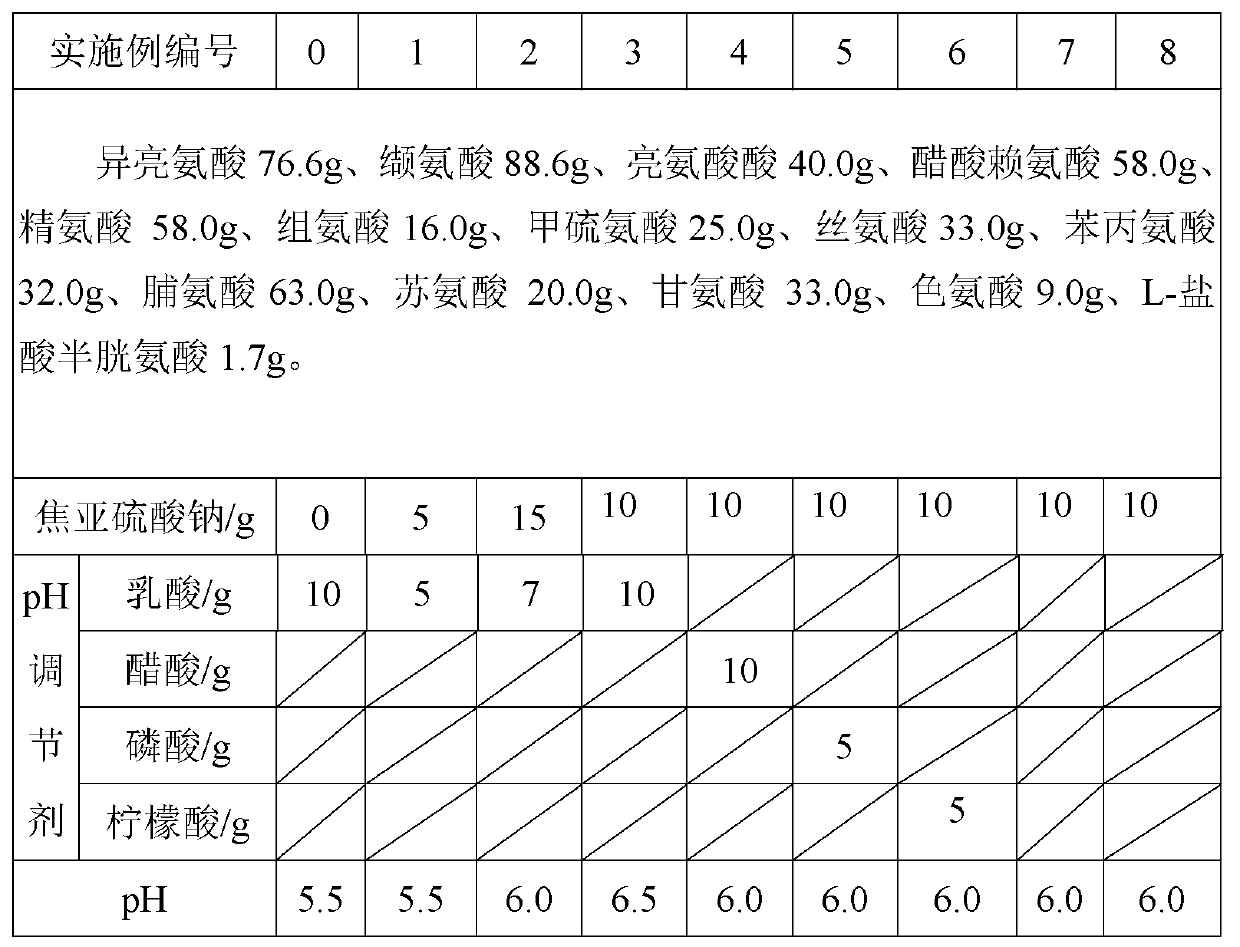
[0014] In the embodiment, the injection is prepared in 10000ml, and the subpackage adopts 250ml glass bottles. Sterilization is performed by autoclaving at 105°C for 40 minutes. After adding the pH regulator, use 0.1M sodium hydroxide solution or hydrochloric acid to adjust the pH to 5.5-6.5 (if the pH is qualified, it is not necessary to add more).
[0015] Described injection adopts following method to prepare
[0016] 1) Take 70% to 75% of the prescribed amount of water for injection, heat it to 45-50°C under nitrogen protection, add lysine acetate, arginine, and histidine and stir until completely dissolved, add the prescribed amount of lactic acid or For other pH regulators, use hydrochloric acid to adjust the pH value to 5.0~5.5, and then add tryptophan, cysteine hydrochloride, leucine, isoleucine, valine, methionine, threonine, alanine Amino acid, proline, serine, glycine, phenylalanine, and sodium metabisulfite are fully stirred to dissolve completely, and water fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com