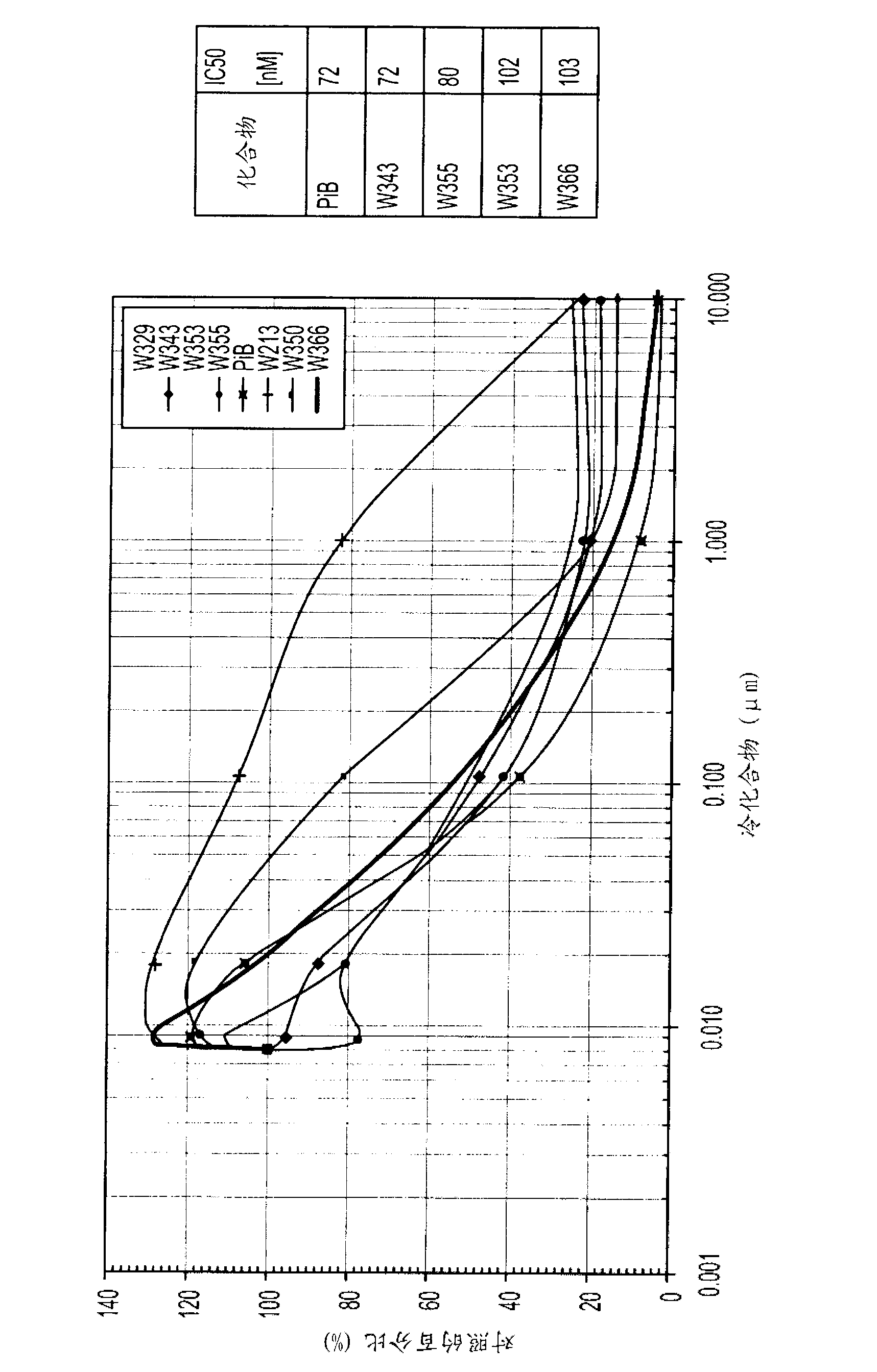
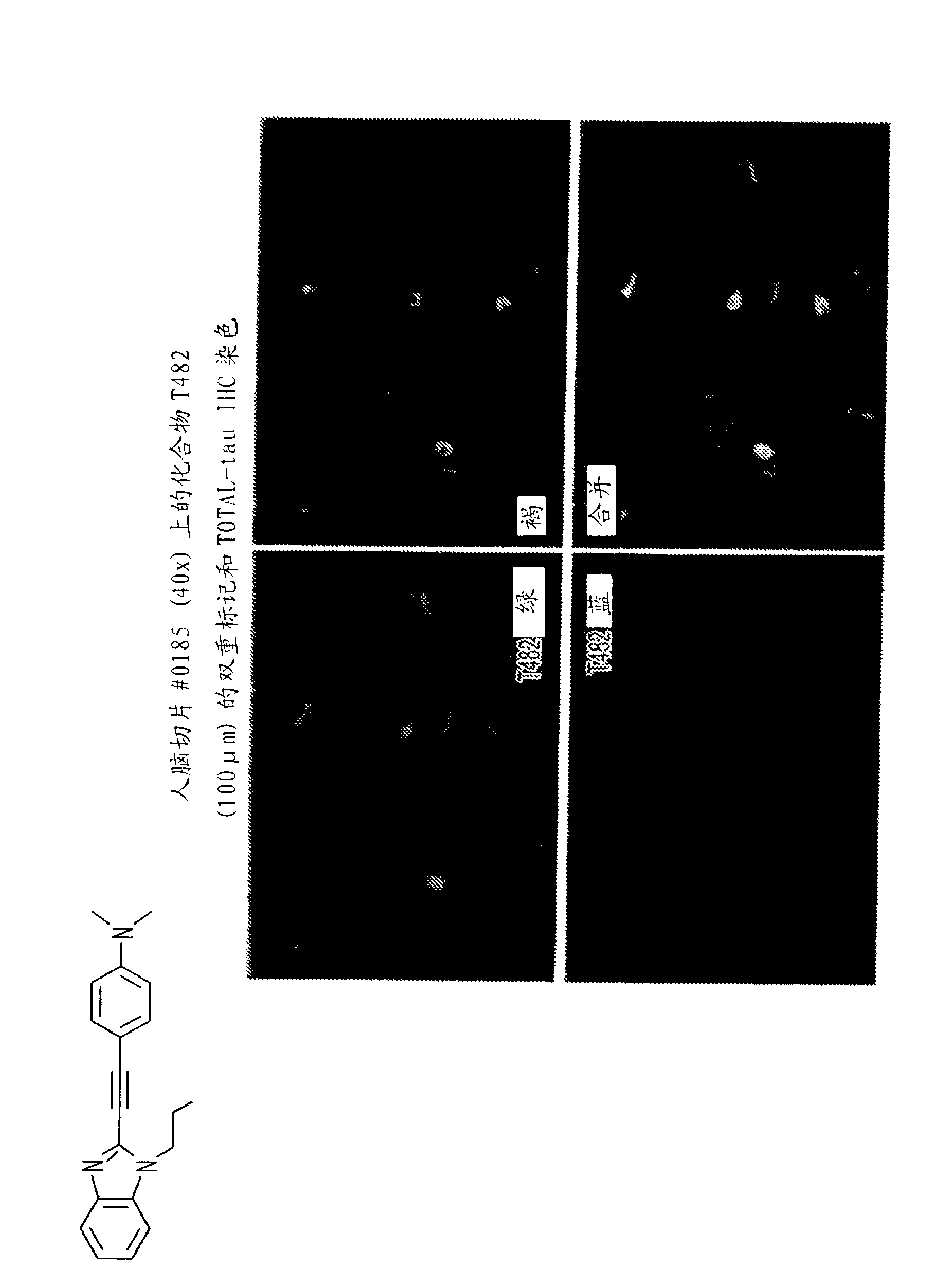
Imaging agents for detecting neurological disorders
A technology of medicinal salts and compounds, applied in the field of imaging agents for detecting neurological disorders, which can solve the problems of patients confirming AD and not solving the problem of understanding connections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0076] The following description will describe the invention and its advantageous embodiments. The invention is not in any way limited to these advantageous embodiments, since the latter description is only for the purpose of exemplifying the invention, which is intended to embrace possible variations and modifications, as those skilled in the art do without departing from the scope of the invention. understandable below.
[0077] One embodiment of the present invention relates to compounds of general formula (I)
[0078]
[0079] in
[0080] A is the key, (C 1 -C 4 ) Alkyl, (C 3 -C 6 ) cycloalkyl, (C 2 -C 4 ) ene or (C 1 -C 4 ) alkyne;
[0081] Z is an aryl group selected from:
[0082]
[0083] in
[0084] x 1 and x 13 each independently is C, CH, N, O or S;
[0085] x 2 –X 12 and x 14 -X 18 each independently is C, CH or N;
[0086] Y 1 is N, O or S;
[0087] R 1 -R 2 Each independently is H, halogen, hydroxyl, nitro, cyano, amino, alkyl, alkox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com