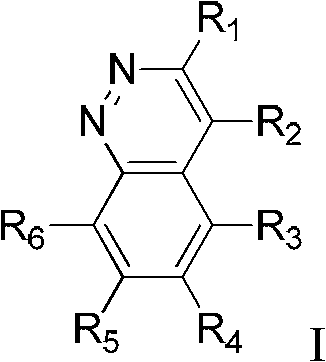
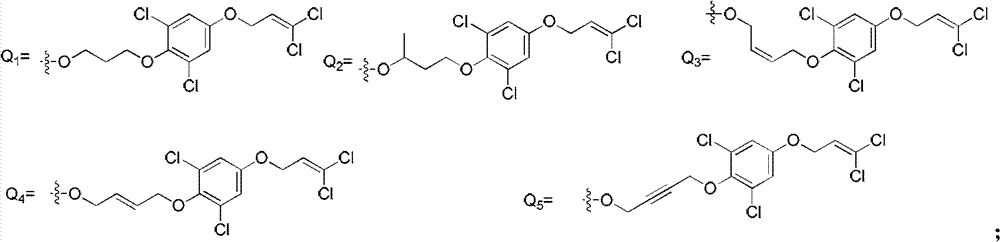
A kind of ether compound containing cinnoline ring and its application
A technology of ether compound and cinnoline ring, applied in the field of ether compound containing cinnoline ring, can solve the problem that the insecticidal activity of ether compound is not disclosed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0059] The preparation of example 1 compound 2:
[0060]
[0061] Add 3-(2,6-dichloro-4-(3,3-dichloro-allyloxy)phenoxy)propan-1-ol (0.69 g, 2.00 mmol, synthetic method refer to Example 1, 2, 3) in the patent CN1860874A), tetrahydrofuran (15mL), sodium hydride (0.10 g, 4.00 mmol), stirred until no bubbles were released, added 3,7-dichlorocinnoline (0.40 g, 2.00 mmol , the synthesis method refers to Journal of Medicinal Chemistry, 2010, 53(8): 3330-3348), and reacted overnight at room temperature. After the reaction was complete, the reaction solution was put into water (50mL), extracted with ethyl acetate (3×100mL), the organic layer was washed with saturated sodium bicarbonate and saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. got leftovers. The residue was purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:1.5) to obtain 0.28 g of compound 2, a yellow solid, yield 26%,...
example 2
[0062] The preparation of example 2 compound 4:
[0063]
[0064] Add 3-(2,6-dichloro-4-(3,3-dichloro-allyloxy)phenoxy)propan-1-ol (0.35 g, 1.00 mmol), tetrahydrofuran (10 mL ), sodium hydride (0.14 g, 2.00 mmol), stirred until no bubbles are released, added 4-chlorocinnoline (0.16 g, 2.00 mmol, synthetic method with reference to Journal of Organic Chemistry, 1946, 11:419-428), React overnight at room temperature. After the reaction was complete, the reaction solution was put into water (50mL), extracted with ethyl acetate (3×100mL), the organic layer was washed with saturated sodium bicarbonate and saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. got leftovers. The residue was purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:2) to obtain 0.08 g of compound 4, a yellow solid, yield 6%, melting point: 96-98°C
example 3
[0065] The preparation of example 3 compound 7:
[0066]
[0067] Add 3-(2,6-dichloro-4-(3,3-dichloro-allyloxy)phenoxy)propan-1-ol (0.50 g, 1.44 mmol), tetrahydrofuran ( 15mL), sodium hydride (0.05 g, 1.44 mmol), stirred until no bubbles were emitted, added 4-chloro-7-trifluoromethylcinnoline (0.33 g, 1.44 mmol, the synthetic method refers to Journal of Organic Chemistry, 1946 , 11:419-428), react overnight at room temperature. After the reaction was complete, the reaction solution was put into water (50mL), extracted with ethyl acetate (3×100mL), and the organic layer was washed with saturated sodium bicarbonate (50mL) and saturated sodium chloride solution (50mL), and dried over anhydrous magnesium sulfate. , filtered, and concentrated under reduced pressure to obtain a residue. The residue was purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:3) to obtain 0.18 g of compound 7, a yellow solid, yield 23%, melting point: 85-87°C
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com