4-(chloromethyl)-7-hydroxy coumarin compound and preparation method thereof
A technology of hydroxycoumarin and chloromethyl, which is applied in the field of compound 4--7-hydroxycoumarin and its preparation, can solve the problems of high toxicity and side effects, difficulty in controlling the quality of Chinese patent medicines, and difficulty in accurately evaluating clinical efficacy. To achieve the effect of scientific production methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] Below in conjunction with embodiment, the technical solution of the present invention is further described, and following embodiment is illustrative, not limiting, can not limit protection scope of the present invention with following embodiment.
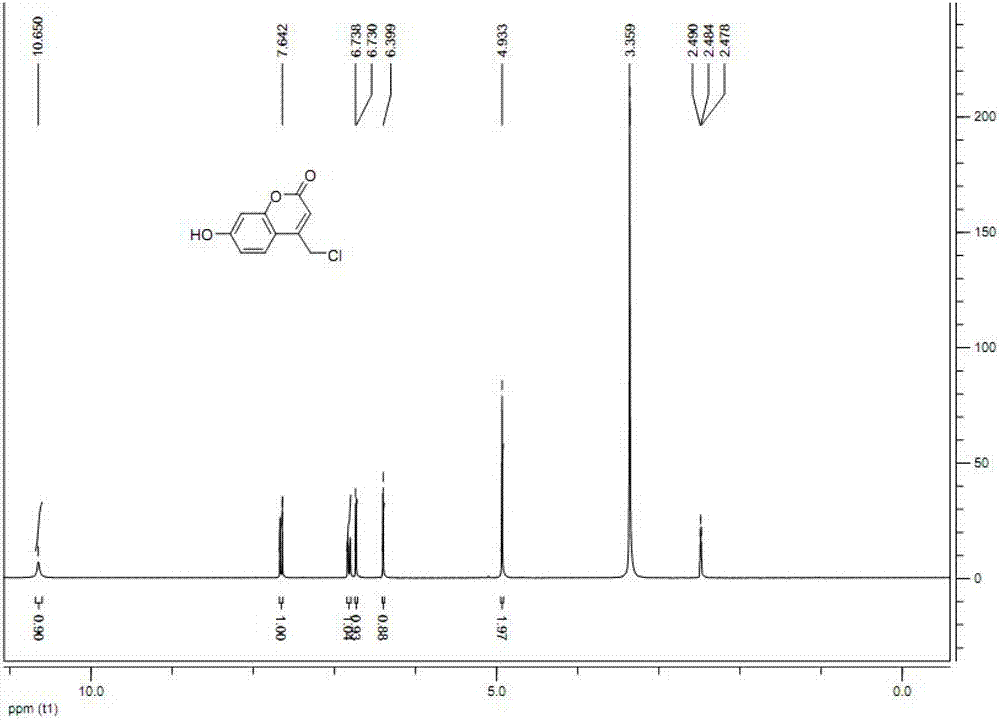
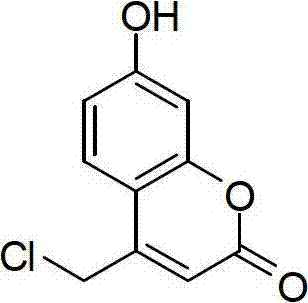
[0018] The structural formula of 4-(chloromethyl)-7-hydroxycoumarin prepared by the present invention is:
[0019]
[0020] reaction route
[0021]
[0022] Raw materials: resorcinol, ethyl chloroacetoacetate, and concentrated sulfuric acid are commercially available
[0023] The synthetic method of 4-(chloromethyl)-7-hydroxycoumarin is as follows:
[0024] Weigh resorcinol (0.5mol), slowly add to 90ml concentrated H under cooling 2 SO 4 , stirred continuously, then added ethyl chloroacetoacetate (0.5mol), turned to room temperature and stirred overnight, slowly poured the reaction solution into 10 times the amount of ice-water bath, stirred at room temperature for 1 hour, a large amount of solids were produced, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com