Method for preparing and purifying dabigatran etexilate intermediate
A compound, the technology of ethyl propionate, which is applied in the field of preparation and purification of dabigatran etexilate intermediates, can solve the problems of no yield and purity, and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
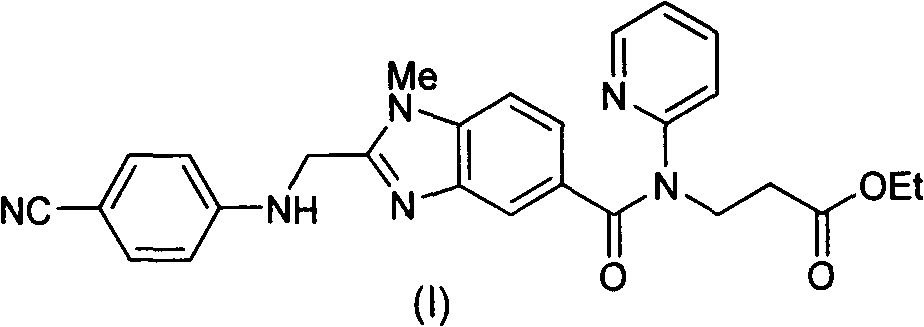
[0048] Example 1: 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazole-5-[N-(2-pyridyl)formamido]}-propionic acid Synthesis of ethyl ester (I)
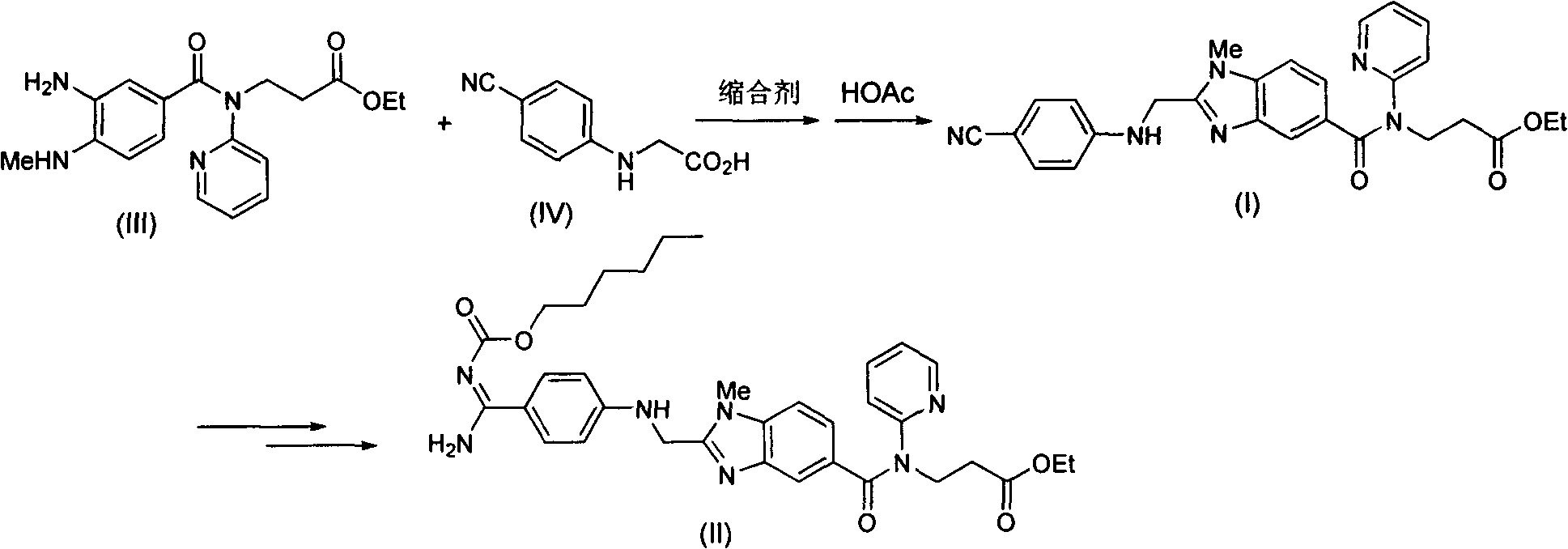
[0049] Add 687g of 2-(4-cyanoanilino)acetic acid and 10L of tetrahydrofuran into the reaction flask, add 632g of carbonyldiimidazole under stirring, and stir at room temperature for 1 hour. Add 1334g of 3-[4-methylamino-3-amino-N-(2-pyridyl)-benzamido]-acrylic acid ethyl ester (III), stir at room temperature for 12 hours, the raw material disappears and a condensate intermediate is formed. Tetrahydrofuran was removed under reduced pressure, and 10 L of ethyl acetate was added. Wash with saturated potassium carbonate solution, extract and separate the organic phase. Add 468g of glacial acetic acid, reflux reaction for 12 hours, the condensate intermediate disappears, and the ethyl acetate solution of the obtained compound (I) is washed with a saturated potassium carbonate solution, and then directly proceeds to the next step of react...
Embodiment 2
[0051] Example 2: 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazole-5-[N-(2-pyridyl)formamido]}-propionic acid Synthesis of Ethyl Estersuccinate (V)
[0052] Add 460 g of succinic acid to the ethyl acetate solution containing compound (I) in Example 1, and stir at room temperature for 3 hours. The precipitated solid was filtered out and dried to obtain 2015 g of compound (V), with a two-step total yield of 86% and a purity of 99%.
[0053] 1 H NMR (400MHz, d-DMSO) δ1.10(t, J=7.2Hz, 3H), 2.39(s, 4H), 2.65(t, J=7.2Hz, 2H), 3.73(s, 3H), 3.95 (q, J=7.2Hz, 2H), 4.20(t, J=7.2Hz, 2H), 4.57(d, J=5.6Hz, 2H), 6.79(d, J=8.0Hz, 2H), 6.87(d , J=8.0Hz, 1H), 7.09(dd, J=4.8, 7.2Hz, 1H), 7.14(d, J=8.0, 1H), 7.22(t, J=4.8, 1H), 7.38(d, J =8.0, 1H), 7.42-7.46(m, 3H), 7.52(t, J=8.0Hz, 1H), 8.36(d, J=2.8, 1H), 12.11(brs, 2H)
Embodiment 3
[0054] Example 3 3-{2-[(4-cyanoanilino)methyl]-1-methyl-benzimidazole-5-[N-(2-pyridyl)formamido]}-propionic acid ethyl Preparation of pure ester (I)
[0055] 1000 g of compound (V) obtained in Example 2 was added to a mixed solvent composed of 3 L of ethyl acetate and 3 L of water. Add 230 g of potassium carbonate and stir at room temperature for 1 hour. Separate the liquid to obtain the organic phase. After the organic phase was dried, the organic solvent was removed under reduced pressure to obtain 780 g of pure compound (I), with a yield of 97% and a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com