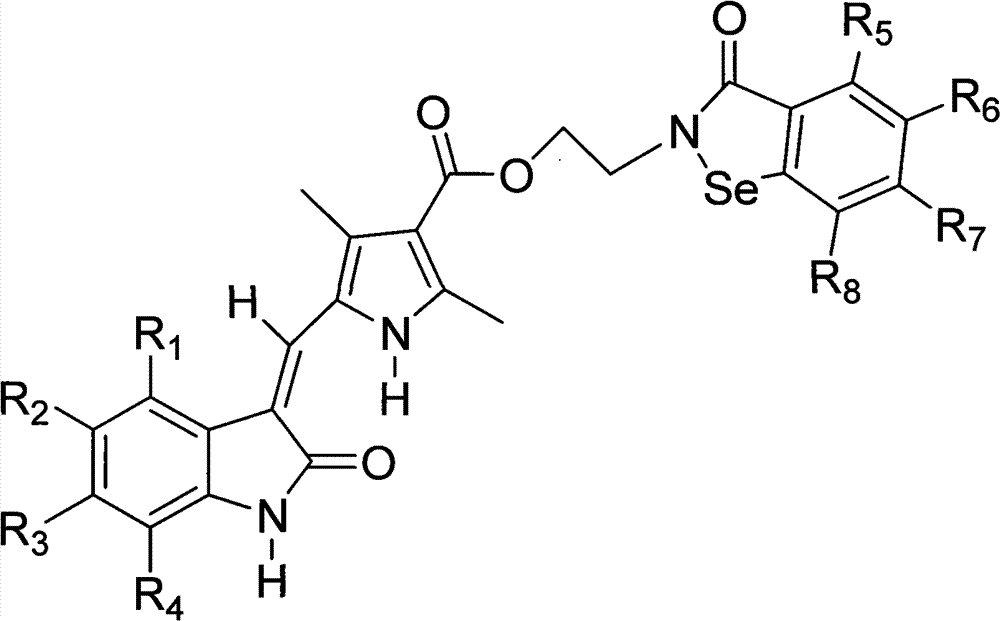
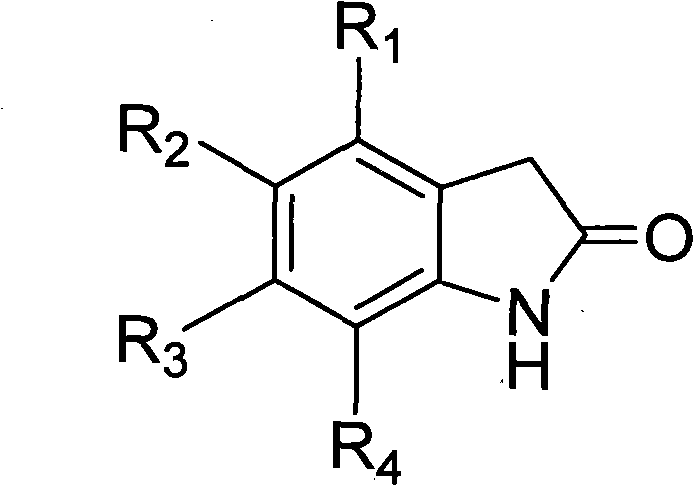
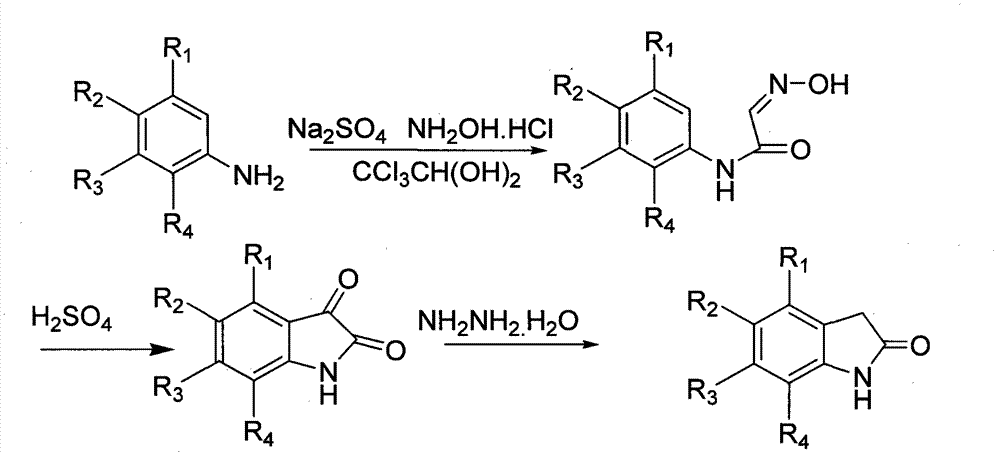
Benzisoselenazolone-modified pyrrolyl formate-substituted indolone compound and use thereof
A technology of benzisoselenazole and compounds, applied in the field of chemistry, can solve problems such as blood toxicity and increased risk of hypertension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] The preparation of embodiment 1 N-phenyl-2-oximinoacetamide
[0110]
[0111] After anhydrous sodium sulfate (35g, 246mmol) was added in batches to a solution of chloral hydrate (5g, 30.5mmol) in water (90ml) at 40°C, aniline (2.57g, 27.6mmol) and 36% concentrated hydrochloric acid (3ml , 36mmol) in water (20ml) solution, then dropwise into hydroxylamine hydrochloride (6.1g, 88.4mmol) in water (27.5ml) solution, after dropping, the temperature was raised to 85°C for 2h, cooled to 50°C, suction filtered, washed with cold water, White flocculent solid (3.53 g, 78%) dried from filter cake.
Embodiment 2
[0112] The preparation of embodiment 2 isatin
[0113]
[0114] 98% concentrated sulfuric acid (46ml) was heated to 60°C, and N-phenyl-2-oximinoacetamide (1.8g, 11mmol) was added in batches under stirring, and the addition was completed within 0.5h. After the addition, the temperature was raised to 80°C for 15min. After cooling to room temperature, it was poured into 250 g of crushed ice, stirred at room temperature for 0.5 h, filtered with suction, and washed with ice water to obtain an orange solid (1.2 g, 75%).
[0115] 1 H NMR (400MHz, DMSO-d 6 ): 6.89(d, 1H), 7.04(m, 1H), 7.48(d, 1H), 7.57(m, 1H), 11.02(s, br, 1H)
Embodiment 3
[0116] Example 3 Preparation of 1,3-dihydroindolin-2-one
[0117]
[0118] Weigh isatin (0.8g, 5.4mmol), first add 0.3g of it into 80% hydrazine hydrate (10ml), heat to reflux at 100°C, add the remaining 0.5g in batches, complete the addition within 2h, and heat up to 110°C Continue to reflux for 4h, cool to room temperature, add dropwise 36% concentrated hydrochloric acid to adjust to pH = 3, continue to stir at room temperature for 12h, a solid precipitates, suction filters, washes with water to give a brown solid (0.44g, 61%).
[0119] 1 H NMR (400MHz, DMSO-d 6 ): 3.44(s, 2H), 6.79(d, 1H), 6.90(t, 1H), 7.13(d, 1H), 7.18(d, 1H), 10.33(s, br, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com