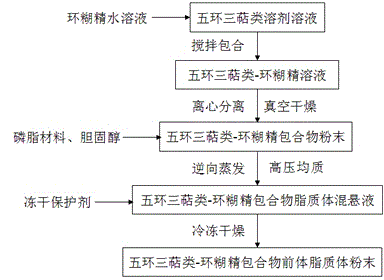
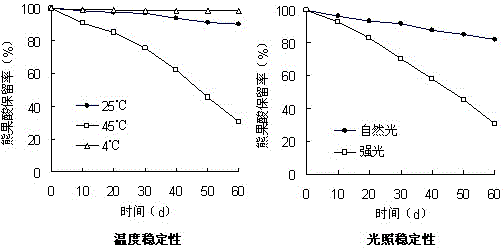
Pentacyclic triterpenoid cyclodextrin clathrate compound proliposome and preparation method thereof
A technology of cyclodextrin inclusion compound and pentacyclic triterpenoids, which is applied in the field of pentacyclic triterpenoid compound cyclodextrin inclusion compound proliposome and its preparation, and can solve the problem of low encapsulation rate and poor stability and other problems, to achieve the effect of high encapsulation rate, small particle size and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of ursolic acid β-cyclodextrin inclusion compound proliposome
[0045] Preparation of ursolic acid β-cyclodextrin inclusion compound: Weigh 3.75g of β-cyclodextrin, add 100ml of distilled water to fully dissolve it, take another 0.50g of ursolic acid, dissolve it with 20ml of 95% ethanol and slowly Add it dropwise to the above aqueous solution of β-cyclodextrin, stir at 30°C for 6 hours, centrifuge the dispersion of the inclusion complex at 70000g for 60 minutes, the supernatant is the aqueous solution of the inclusion compound of ursolic acid β-cyclodextrin. The clear liquid was concentrated in vacuum, and the concentrated liquid was dried in a vacuum oven at 40°C and 0.08 MPa for 6 hours to obtain the inclusion compound of ursolic acid β-cyclodextrin in the form of a white powder, and the inclusion rate reached 82.34% by measurement.
[0046] Determination of ursolic acid content: Determination of ursolic acid by high performance liquid c...
Embodiment 2
[0052] Embodiment 2: Preparation of ursolic acid hydroxypropyl-beta-cyclodextrin inclusion compound proliposome
[0053] Preparation of ursolic acid hydroxypropyl-β-cyclodextrin inclusion complex: Weigh 8.00g of hydroxypropyl-β-cyclodextrin, add 100ml of distilled water to fully dissolve it, and take another ursolic acid 0.38g, Dissolve in 20ml of 95% ethanol and slowly add it dropwise to the above β-cyclodextrin aqueous solution, stir at 20°C for 3 hours, centrifuge the dispersion of inclusion complex at 70000g for 60 minutes, the supernatant is ursolic acid hydroxypropyl-β -Aqueous solution of cyclodextrin inclusion complex, the supernatant is concentrated in vacuum, and the concentrated solution is dried in a vacuum oven at 40°C and 0.08MPa for 6 hours to obtain ursolic acid hydroxypropyl-β-cyclodextrin inclusion compound in the form of white powder The inclusion rate was determined to reach 79.08%.
[0054] 20.00g of hydrogenated soybean lecithin and 5.50g of cholesterol ...
Embodiment 3
[0056] Example 3: Preparation of oleanolic acid β-cyclodextrin inclusion complex proliposome
[0057] Preparation of oleanolic acid β-cyclodextrin inclusion compound: Weigh 4.80g of β-cyclodextrin, add 100ml of distilled water to fully dissolve it, take another 0.70g of oleanolic acid, and dissolve it in 20ml of 95% ethanol Then slowly add it dropwise to the above-mentioned β-cyclodextrin aqueous solution, stir at 60°C for 4 hours, then centrifuge the dispersion of the clathrate at 70000g for 60min, the supernatant is the aqueous solution of the ursolic acid β-cyclodextrin clathrate, The supernatant was concentrated in vacuum, and the concentrated solution was dried in a vacuum drying oven at 40°C and 0.08 MPa for 6 hours to obtain the inclusion compound of ursolic acid β-cyclodextrin in the form of white powder, and the inclusion rate reached 86.27% by measurement.
[0058] Determination of oleanolic acid content: Determination of ursolic acid by high performance liquid chrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com