Preparation method of thiosulfuric acid derivative for rubber industry
A technology of thiosulfuric acid and rubber industry, which is applied in the direction of organic chemistry, can solve the problems of reducing the safety of the production process and the volatilization of organic solvents, and achieve the solution of acidification of the solution, improvement of product purity, and simple and easy preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
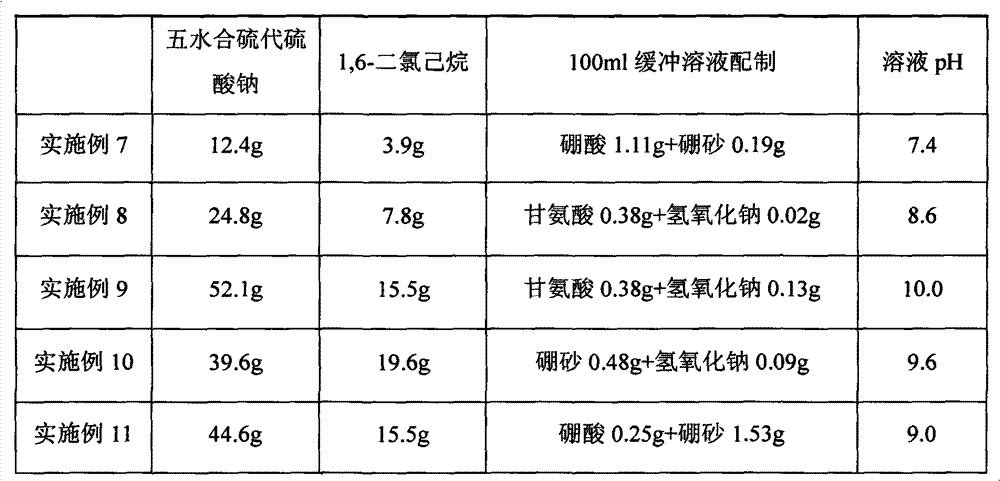
Examples
Embodiment 1
[0024] A 200mL four-necked flask equipped with a mechanical stirring device, a thermometer and a reflux condenser was used as a reaction vessel, and 0.42g of sodium bicarbonate and 0.04g of sodium hydroxide were weighed, and 110mL of water was added to prepare sodium bicarbonate with a pH value of 9.6- Sodium hydroxide buffer solution was placed in a reaction vessel, and 13.6g of sodium thiosulfate pentahydrate was weighed and added to the above solution. After mechanical stirring and dissolving, 6.7g of 1,6-dibromohexane was added while stirring, and the temperature was raised and heated. For the above solution, the temperature of the reaction solution was raised to 102°C. After stirring and reacting for 5 hours under reflux, the solution was poured out and 110 mL of ethanol was added, and the solution was left to stand at 4°C for 3 hours. A large amount of solids precipitated, and the solid product was filtered out. After drying, dihydrate hexamethylene 1,6-dithiosulfate diso...
Embodiment 2
[0026] A 200mL four-necked flask equipped with a mechanical stirring device, a thermometer and a reflux condenser was used as a reaction vessel, and 0.42g of sodium bicarbonate and 0.04g of sodium hydroxide were weighed, and 110mL of water was added to prepare sodium bicarbonate with a pH value of 9.6- Sodium hydroxide buffer solution was placed in a reaction vessel, and 23.6g of ammonium thiosulfate was weighed and added to the above solution. After mechanical stirring and dissolving, 12.4g of 1,6-dichlorohexane was added while stirring, and the temperature was raised to heat the above solution , raise the temperature of the reaction solution to 80°C, stir and react under reflux for 6h, pour out the solution and add 200mL of ethanol, leave the solution at 0°C for 2h, a large amount of solid precipitates, filter out the solid product, and dry it at 55°C That is, hexamethylene dihydrate 1,6-diammonium thiosulfate was obtained.
Embodiment 3
[0028] A 200mL four-necked flask equipped with a mechanical stirring device, a thermometer and a reflux condenser was used as a reaction vessel, and 0.42g of sodium bicarbonate and 0.09g of sodium hydroxide were weighed, and 110mL of water was added to prepare a sodium bicarbonate with a pH value of 10.0- Sodium hydroxide buffer solution was placed in a reaction vessel, and 69.5g of sodium thiosulfate pentahydrate was weighed and added to the above solution. After mechanical stirring and dissolving, 24.1g of 1,6-dichlorohexane was added while stirring, and the temperature was raised and heated. For the above solution, raise the temperature of the reaction solution to 90°C, stir and react under reflux for 9h, then pour out the solution and add 600mL of ethanol, leave the solution at 10°C for 5h, a large amount of solids precipitate, filter out the solid product, 50°C After drying, dihydrate hexamethylene 1,6-dithiosulfate disodium salt is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com