Light-driven regulation preparation method and electrocatalysis activity of nanometer material
A nanomaterial, light-driven technology, used in chemical instruments and methods, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve the problems of long reaction time and organic solvents, high temperature, lack of universality, etc. The effect of excellent activity, mild conditions, and excellent catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
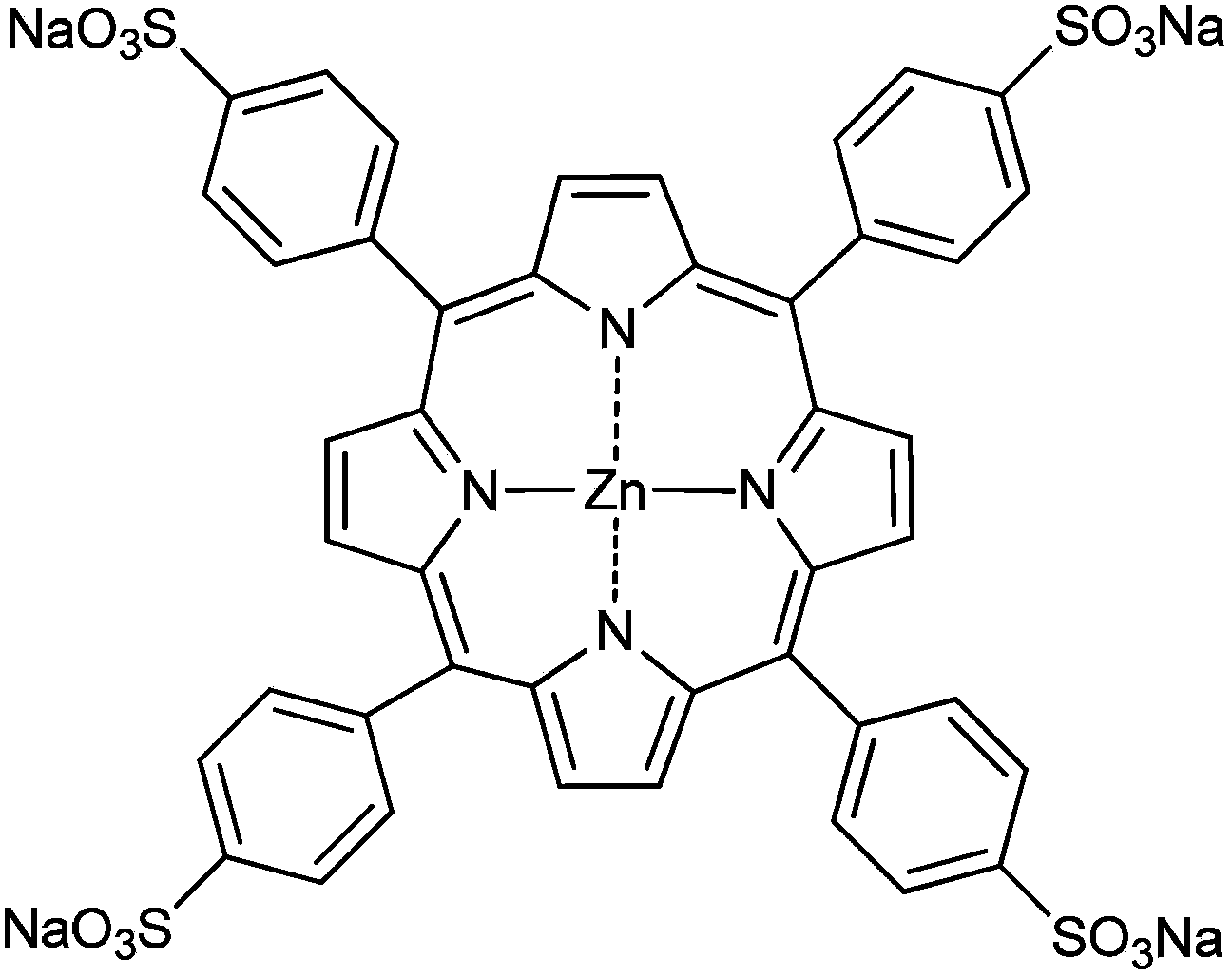
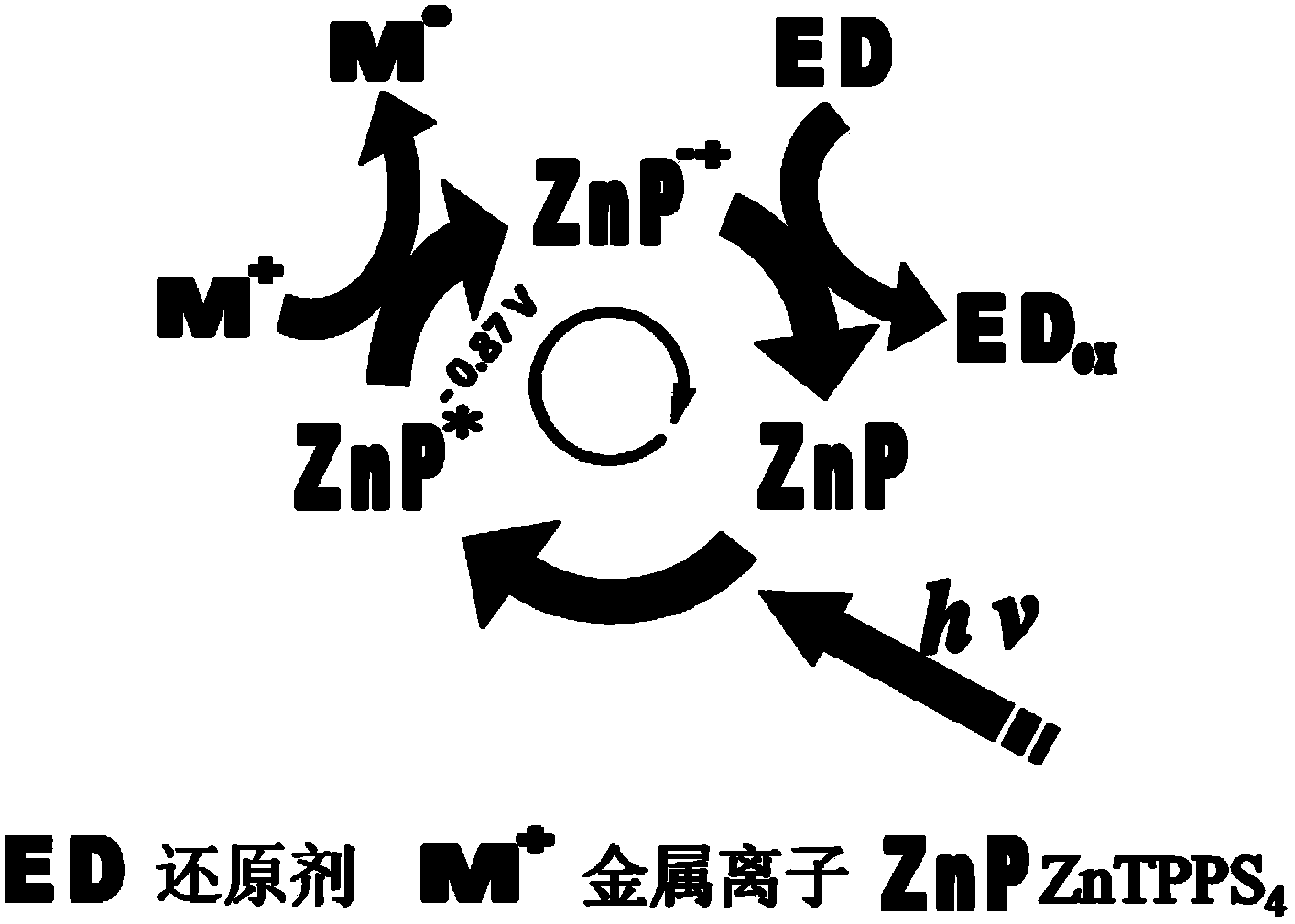
[0055] Pipette 1 mL of zinc porphyrin (10 μM) polyoxyethylene lauryl ether (Brij-35, 1 mM) aqueous solution into the reaction flask, and the solution was in a stirring state, followed by adding 1 mL of 20 mM potassium chloroplatinite aqueous solution and 1 mL of 150 mM Aqueous solution of ascorbic acid, reacted in a tungsten halogen lamp (75V, light intensity 570nmol cm -2 the s -1 ) under irradiation for 60 seconds, turn off the light source, and the reaction is complete after at least 30 minutes. The reacted mixture is transferred to a centrifuge test tube, centrifuged to obtain a solid product, washed with deionized water, and dried to obtain a spherical branched platinum nano-catalyst.
[0056] Such as image 3 , TEM photos show that the spherical branched structure has a single shape and a uniform size.
[0057] Such as Figure 4 , the particle size distribution shows that the average particle size is 15.61±1.96nm, and the particle size distribution is 12.56%.
[005...
Embodiment 2
[0063] Embodiment 2: (lighting time is different)
[0064] Pipette 1 mL of zinc porphyrin (10 μM) polyoxyethylene lauroyl ether (Brij-35, 1 mM) aqueous solution into the reaction flask, and the solution was in a stirring state, followed by adding 1 mL of 20 mM potassium chloroplatinite solution and 1 mL of 150 mM Ascorbic acid solution, reacted in a tungsten halogen lamp (75V, light intensity 570nmol cm -2 the s -1 ) for 20 seconds under irradiation, turn off the light source, and the reaction is complete after at least 30 minutes. The reacted mixture is transferred to a centrifuge test tube, centrifuged to obtain a solid product, washed with deionized water, and dried to obtain a spherical branched platinum nano-catalyst.
[0065] Such as Figure 10 , TEM photos show that the spherical branched structure has a single shape and a uniform size.
[0066] Such as Figure 11 , the particle size distribution shows that the average particle size is 27.82nm±3.64nm, and the parti...
Embodiment 3
[0067] Embodiment 3: (lighting time is different)
[0068] Pipette 1 mL of zinc porphyrin (10 μM) polyoxyethylene lauroyl ether (Brij-35, 1 mM) aqueous solution into the reaction flask, and the solution was in a stirring state, followed by adding 1 mL of 20 mM potassium chloroplatinite solution and 1 mL of 150 mM Ascorbic acid solution, reacted in a tungsten halogen lamp (75V, light intensity 570nmol cm -2 the s -1 ) for 40 seconds under irradiation, turn off the light source, and the reaction is complete after at least 30 minutes. The reacted mixture is transferred to a centrifuge test tube, centrifuged to obtain a solid product, washed with deionized water, and dried to obtain a spherical branched platinum nano-catalyst.
[0069] Such as Figure 12 , TEM photos show that the spherical branched structure has a single shape and a uniform size.
[0070] Such as Figure 13 , the particle size distribution shows that the average particle size is 19.85±2.5nm, and the particle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com