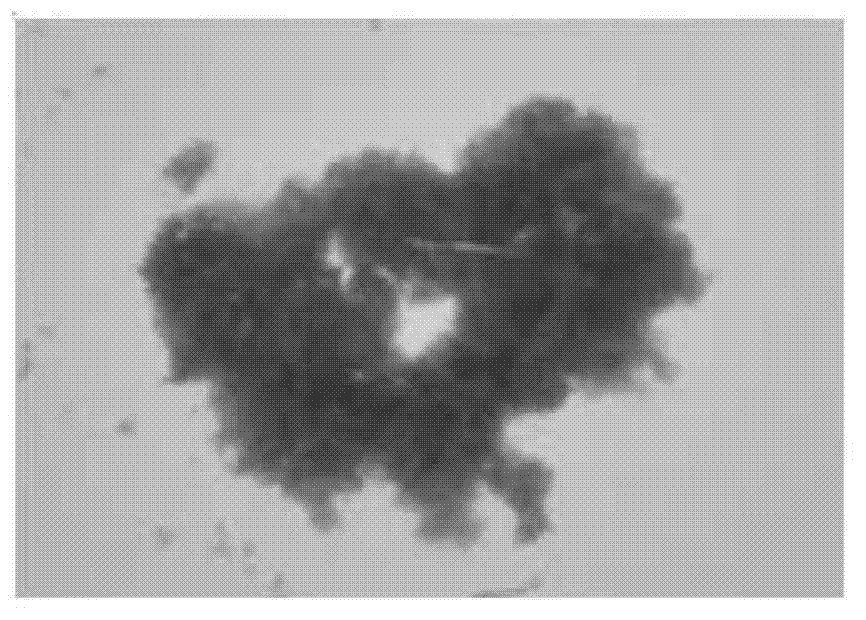
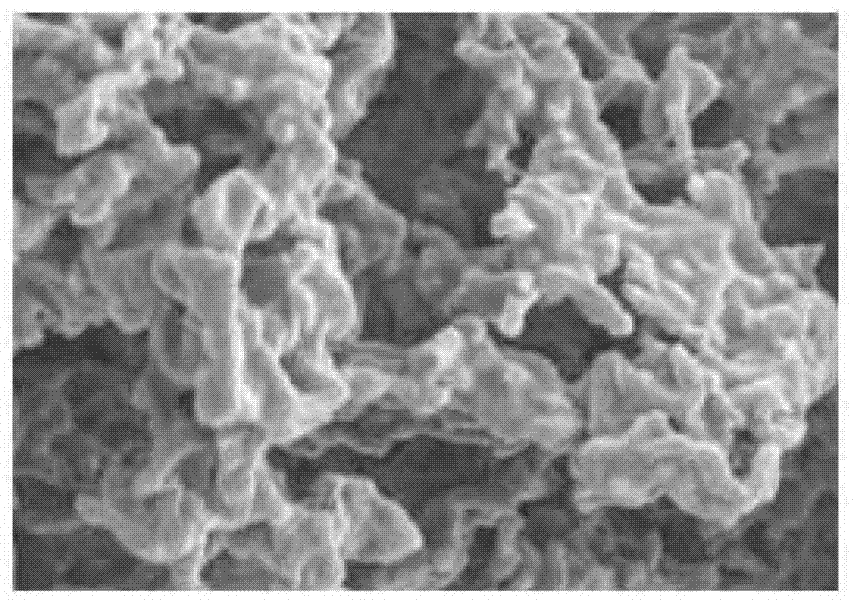
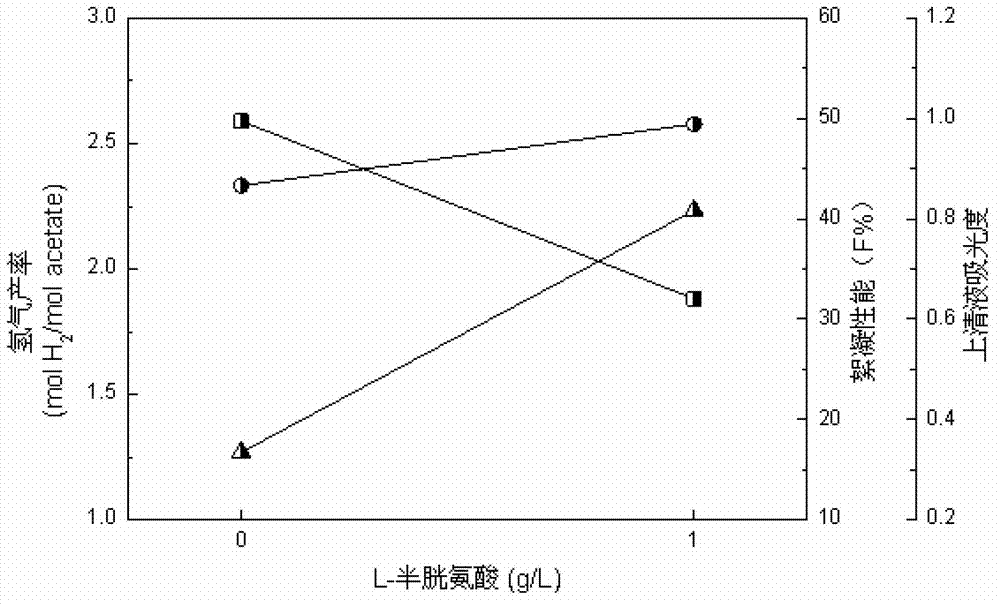
Method for reinforcing flocculation performance of rhodopseudomonas faecalis of light-fermentative bacteria
A Rhodopseudomonas, photo-fermentation technology, applied in microorganism-based methods, bacteria, fermentation and other directions, can solve the problem of low hydrogen production efficiency, biomass loss, and poor flocculation characteristics of photo-fermentative bacteria in photo-fermentation hydrogen-producing reactors. problem, to achieve the effect of flocculation growth, reduction of strain loss, and effective separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0011] Embodiment 1: The method for enhancing the flocculation performance of photofermentation bacteria Rhodopseudomonas faecalis in this embodiment is carried out according to the following steps:
[0012] 1. Inoculate the Rhodopseudomonas faecalis (Rhodopseudomonas faecalis) RLD-53 bacterium solution with a cell concentration of 0.8 to 1.0 g / L into the growth medium at an inoculum size of 10%, then place it on a shaker at 120 rpm, and Cultivate in continuous light at 35°C for 16 hours, and the light intensity is 150W / m 2 , get the seed solution;
[0013] 2. Inoculate the seed solution obtained in step 1 into the hydrogen-producing medium containing 0.75-1.25g / L L-cysteine according to the inoculum amount of 10%, then place it on a shaker at 120rpm, and place it under continuous light at 35°C After culturing for 72 hours, a bacterial suspension containing flocs is obtained.
[0014] Rhodopseudomonas faecalis (Rhodopseudomonas faecalis) RLD-53 described in step 1 of this ...
specific Embodiment approach 2
[0017] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the growth medium described in step one is made of 1g sodium acetate, 1g sodium succinate, 1.0g ammonium chloride, 1g beef extract, 0.5g peptone, 0.2 g sodium chloride, 0.2g magnesium sulfate heptahydrate, 0.012g ferrous sulfate heptahydrate, 0.08g anhydrous calcium chloride, 1mL trace element solution, 0.5g dipotassium hydrogen phosphate, 0.5g potassium dihydrogen phosphate, 0.5g L - Cysteine, 1mL growth factor solution and 1L distilled water, pH 7.0; each liter of trace element solution consists of 0.05mg manganese chloride tetrahydrate, 0.05g copper sulfate pentahydrate, 5mg ferric chloride hexahydrate, 0.5mg Cobalt nitrate hexahydrate, 1mg zinc sulfate heptahydrate, 1mg boric acid and the rest of distilled water; each liter of growth factor solution consists of 0.35g nicotinic acid, 0.1g biotin, 0.3g thiamine hydrochloride, 0.1g calcium pantothenate, 0.2g Composed of p-am...
specific Embodiment approach 3
[0018] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: the hydrogen production medium described in step two is composed of 4.1g sodium acetate, 1.69g sodium glutamate, 0.2g sodium chloride, 0.2g seven Magnesium sulfate hydrochloride, 0.012g ferrous sulfate heptahydrate, 0.08g anhydrous calcium chloride, 1mL trace element solution, 0.5g dipotassium hydrogen phosphate, 0.5g potassium dihydrogen phosphate, 1mL growth factor solution and 1L distilled water; One liter of trace element solution consists of 0.05mg manganese chloride tetrahydrate, 0.05g copper sulfate pentahydrate, 5mg ferric chloride hexahydrate, 0.5mg cobalt nitrate hexahydrate, 1mg zinc sulfate heptahydrate, 1mg boric acid and the rest of distilled water; Growth factor solution consists of 0.35g niacin, 0.1g biotin, 0.3g thiamine hydrochloride, 0.1g calcium pantothenate, 0.2g p-aminobenzoic acid, 0.05g vitamin B12, 0.1g pyridoxammonium hydrochloride and the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com