Purifying method for abiraterone acetate
A technology of abiraterone acetate and a purification method, which is applied in the field of purification of raw material drug abiraterone acetate, can solve the problems of viscous filter cake, high production cost, difficult filtration and the like, and achieves simplified purification process, simple operation and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
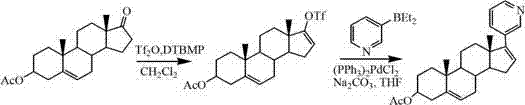
[0024] The preparation of embodiment 1 Abiraterone acetate crude product
[0025] Add 300g (0.91mol) of dehydroepiandrosterone acetate into 2.5L of dichloromethane, stir to dissolve and cool down to 0-10°C, slowly add 183mL (1.08mol) of trifluoromethanesulfonic anhydride dropwise, and stir after the addition is complete After 10 min, 158.6 mL (0.91 mol) of diisopropylethylamine (DIEA) was added dropwise. Insulate and stir for 4 hours, add 2 L and stir thoroughly, separate the layers, extract the aqueous layer with 200 mL of dichloromethane, combine the organic layers and wash with 600 mL×2 water, concentrate to dryness under reduced pressure, add 2.5 L of tetrahydrofuran to dissolve and set aside.
[0026] Add 4.5g (6.4mmol) bistriphenylphosphine palladium dichloride, 120g (0.82mol) diethyl (3-pyridyl) borane, 356g (3.36mol) sodium carbonate and 1600mL water in sequence to the above tetrahydrofuran solution , stirred and refluxed for 2 hours. Then add 1.5 L of water and 1.8 ...
Embodiment 2
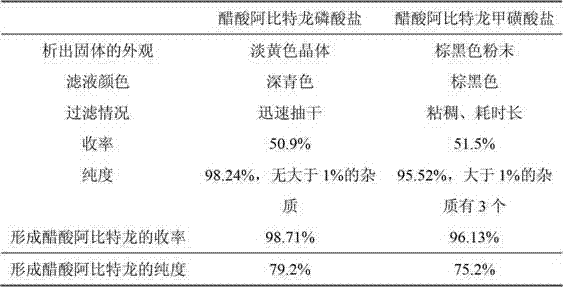
[0027] Purification of Example 2 Abiraterone Acetate Crude Product (phosphoric acid is a salt-forming reagent)
[0028] Add 496.5g of abiraterone acetate crude oil to 2.5L tetrahydrofuran, stir to dissolve, slowly add dropwise 90mL (1.55mol) of phosphoric acid, after the dropwise addition, stir for 10min, stand at room temperature for crystallization for 12h, suction filter, use for filter cake A small amount of tetrahydrofuran was washed and dried to obtain 226.3 g of light yellow crystals, namely abiraterone acetate phosphate, with a yield of 50.9%. HPLC showed a purity of 98.24% and no impurities greater than 1%.
Embodiment 3
[0029] The preparation of embodiment 3 abiraterone acetate (using abiraterone acetate phosphate as raw material)
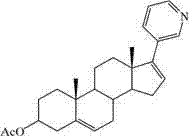
[0030] Add 226.3g (0.46mol) of abiraterone acetate phosphate into 2.5L of dichloromethane, stir evenly, then add 285g (3.4mol) of sodium bicarbonate and 2L of water, after stirring for 2 hours, let it stand for liquid separation, and water the organic phase Wash, dry over anhydrous magnesium sulfate, decolorize with activated carbon, filter, and concentrate to obtain 146.2 g of a white solid, which is abiraterone acetate, with a yield of 79.2%. HPLC shows that the purity is 98.71%, and there are no impurities greater than 1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com