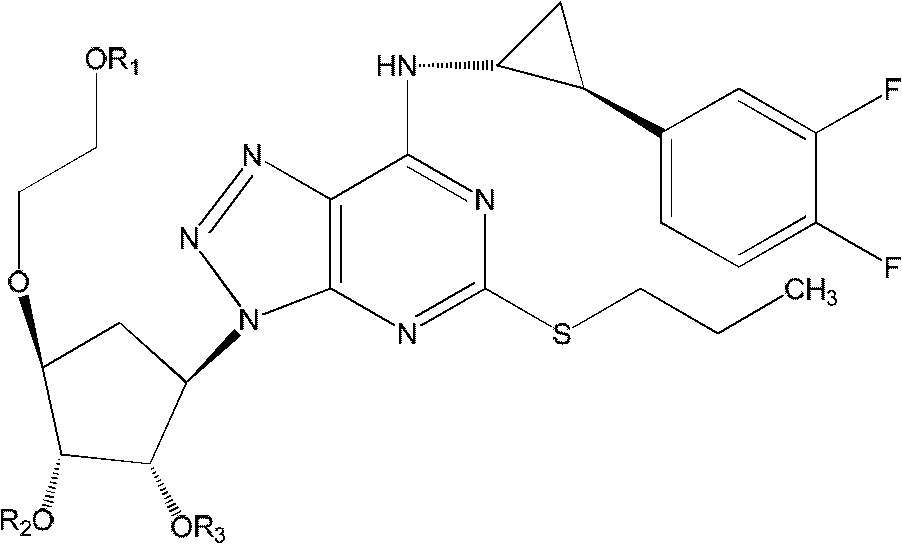
Derivatives of ticagerlor, and preparation method and pharmaceutical application thereof
A technology of ticagrelor and its derivatives, applied in the field of medicine, can solve the problem of inability to accurately control the relative dosage and effect of ticagrelor and aspirin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The synthesis of the L-alanine ester of embodiment 1 ticagrelor
[0072] Step (1), Boc protects the amino group of L-alanine
[0073]
[0074] Dissolve 50g of L-alanine (1.0eq, 0.56mol) in a mixed solution of 100ml of water and 200ml of acetone, add 121.5ml of triethylamine (1.5eq, 0.84mol) under stirring, control the temperature at 25°C, and drop while stirring Add 131.8ml (Boc) 2 O (1.1eq, 0.62mol), stir the reaction for 6h after the dropwise addition, evaporate the acetone under reduced pressure, extract the water layer with diethyl ether, then adjust the pH value of the water layer to 2~3 with dilute hydrochloric acid, and extract the water layer 3 times with ethyl acetate layers, the organic layers were combined, the organic layer was washed with saturated brine, dried and concentrated over anhydrous sodium sulfate to obtain the crude product and recrystallized with petroleum ether and ethyl acetate (2: 1) to obtain 95.4 g of Boc-L-alanine, the yield 90%.
...
Embodiment 2
[0083] The synthesis of the L-phenylalanine ester of embodiment 2 ticagrelor
[0084] Step (1), Boc protects the amino group of L-phenylalanine
[0085]
[0086] Dissolve 50g of L-phenylalanine (1.0eq, 0.30mol) in a mixed solution of 100ml of water and 200ml of acetone, add 65.1ml of triethylamine (1.5eq, 0.45mol) under stirring, control the temperature at 25°C, and stir Add 70.6ml (Boc) dropwise 2O (11eq, 0.33mol), after the dropwise addition, stir the reaction for 6h, evaporate the acetone under reduced pressure, extract the water layer with ether, then adjust the pH value of the water layer to about 2-3 with dilute hydrochloric acid, and extract the water layer 3 times with ethyl acetate Layers, the organic layer was combined, the organic layer was washed with saturated brine, dried and concentrated over anhydrous sodium sulfate to obtain the crude product and recrystallized with petroleum ether and ethyl acetate (2: 1) to obtain 73.9g of Boc-L-phenylalanine, Yield 9...
Embodiment 3
[0094] The synthesis of embodiment 3 ticagrelor L-valine ester
[0095] Step (1), Boc protects the amino group of L-valine
[0096]
[0097] Dissolve 50g of L-valine (1.0eq, 0.426mol) in a mixed solution of 100ml of water and 200ml of acetone, add 92.4ml of triethylamine (1.5eq, 0.64mol) under stirring, control the temperature at 25°C, and drop while stirring Add 100ml (Boc) 2 O (11eq, 0.47mol), after the dropwise addition, stir the reaction for 6h, evaporate the acetone under reduced pressure, extract the water layer with ether, then adjust the pH value of the water layer to about 2-3 with dilute hydrochloric acid, and extract the water layer 3 times with ethyl acetate The organic layer was combined, and the organic layer was washed with saturated brine, dried and concentrated over anhydrous sodium sulfate to obtain the crude product, which was recrystallized with petroleum ether and ethyl acetate (2: 1) to obtain 81.8g of Boc-L-valine. The rate is 88.5%.
[0098] Ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com