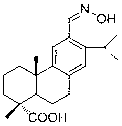
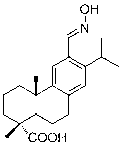
Oximido dehydroabietic acid compound and synthesis method thereof
A technology of dehydroabietic acid and methyl dehydroabietic acid, applied in oxime preparation, organic chemistry, etc., to achieve simple preparation method, excellent physiological activity, and the effect of improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthetic steps of (E)-12-oximino dehydroabietic acid:
[0026] A. Compound ( 1 ) (10.5 g, 0.035 mol) was dissolved in methanol (280 mL), and concentrated H 2 SO 4 (24 mL), over ten minutes, 90 ° C oil bath for about 12 hours of continuous stirring, separation and purification to obtain 9.9 g colorless transparent crystals, that is, the compound ( 2 ), yield 90.4%;
[0027] B. Compound ( 2 ) (500 mg, 1.59 mmol) was dissolved in nitrobenzene (8 mL), and aluminum trichloride (1.1 g, 8.25 mmol) and dichloromethyl methyl ether (0.19 mL, 2.07 mmol) were added successively under stirring, at room temperature Under stirring for 10 hours, separation and purification after post-treatment gave 304 mg of slightly yellowish transparent oily substance, i.e. the compound ( 3 ), yield 55.8%;
[0028] C. Compound ( 3 ) (200 mg, 0.58 mmol) was dissolved in ethanol (8 mL), and pyridine (0.13 mL) was added, followed by the dropwise addition of ethanol solution of ...
Embodiment 2
[0039] Embodiment 2: the synthetic steps of (Z)-12-oximino dehydroabietic acid:
[0040] A. Compound ( 1 ) (10.5 g, 0.035 mol) was dissolved in methanol (280 mL), and concentrated H 2 SO 4 (24 mL), over ten minutes, 90 ° C oil bath for about 12 hours of continuous stirring, separation and purification to obtain 9.9 g colorless transparent crystals, that is, the compound ( 2 ), yield 90.4%;
[0041] B. Compound ( 2) (500 mg, 1.59 mmol) was dissolved in nitrobenzene (8 mL), and aluminum trichloride (1.1 g, 8.25 mmol) and dichloromethyl methyl ether (0.19 mL, 2.07 mmol) were added successively under stirring, at room temperature Under stirring for 10 hours, separation and purification after post-treatment gave 304 mg of slightly yellowish transparent oily substance, i.e. the compound ( 3 ), yield 55.8%;
[0042] C. Compound ( 3 ) (200 mg, 0.58 mmol) was dissolved in ethanol (8 mL), and pyridine (0.13 mL) was added, followed by the dropwise addition of ethanol solution of h...
Embodiment 3
[0053] Embodiment 3: the synthetic steps of (E)-14-oximino dehydroabietic acid:
[0054] A. Compound ( 1 ) (10.5 g, 0.035 mol) was dissolved in methanol (280 mL), and concentrated H 2 SO 4 (24 mL), over ten minutes, 90 ° C oil bath for about 12 hours of continuous stirring, separation and purification to obtain 9.9 g colorless transparent crystals, that is, the compound ( 2 ), yield 90.4%;
[0055] B. Compound ( 2 ) (500 mg, 1.59 mmol) was dissolved in nitrobenzene (8 mL), and aluminum trichloride (1.1 g, 8.25 mmol) and dichloromethyl methyl ether (0.19 mL, 2.07 mmol) were added successively under stirring, at room temperature Under stirring for 10 hours, separation and purification after post-treatment gave 98 mg of slightly yellowish transparent oily substance, namely the compound ( 4 ), yield 18.0%;
[0056] C. Compound ( 4 ) (200 mg, 0.58 mmol) was dissolved in ethanol (8 mL), and pyridine (0.13 mL) was added, followed by the dropwise addition of ethanol solution of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com