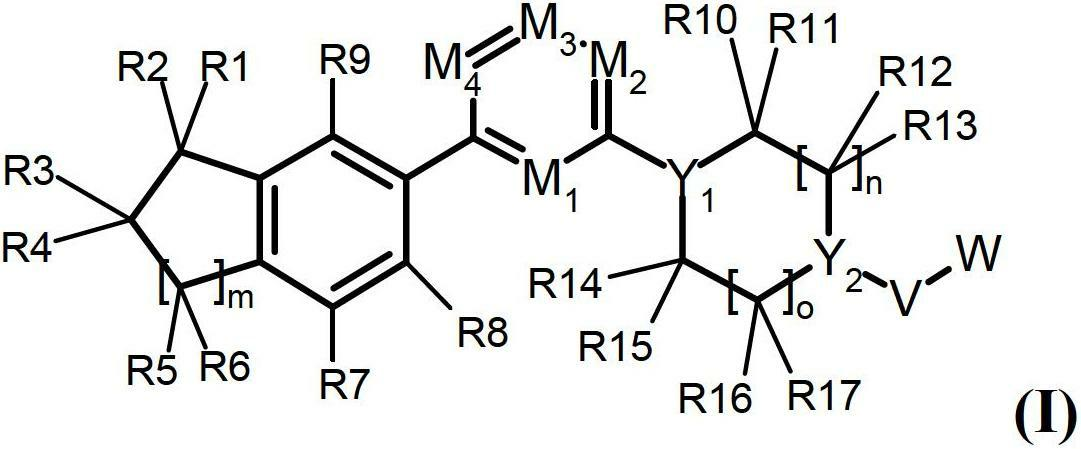
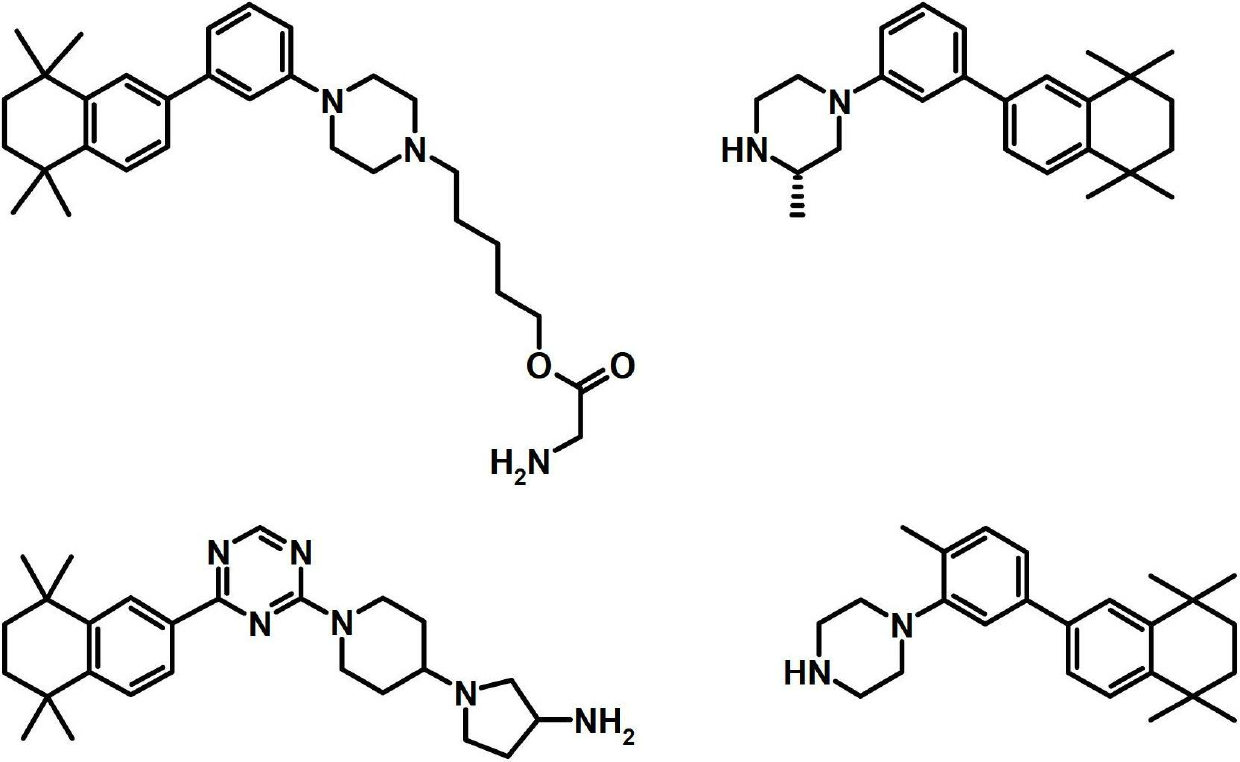
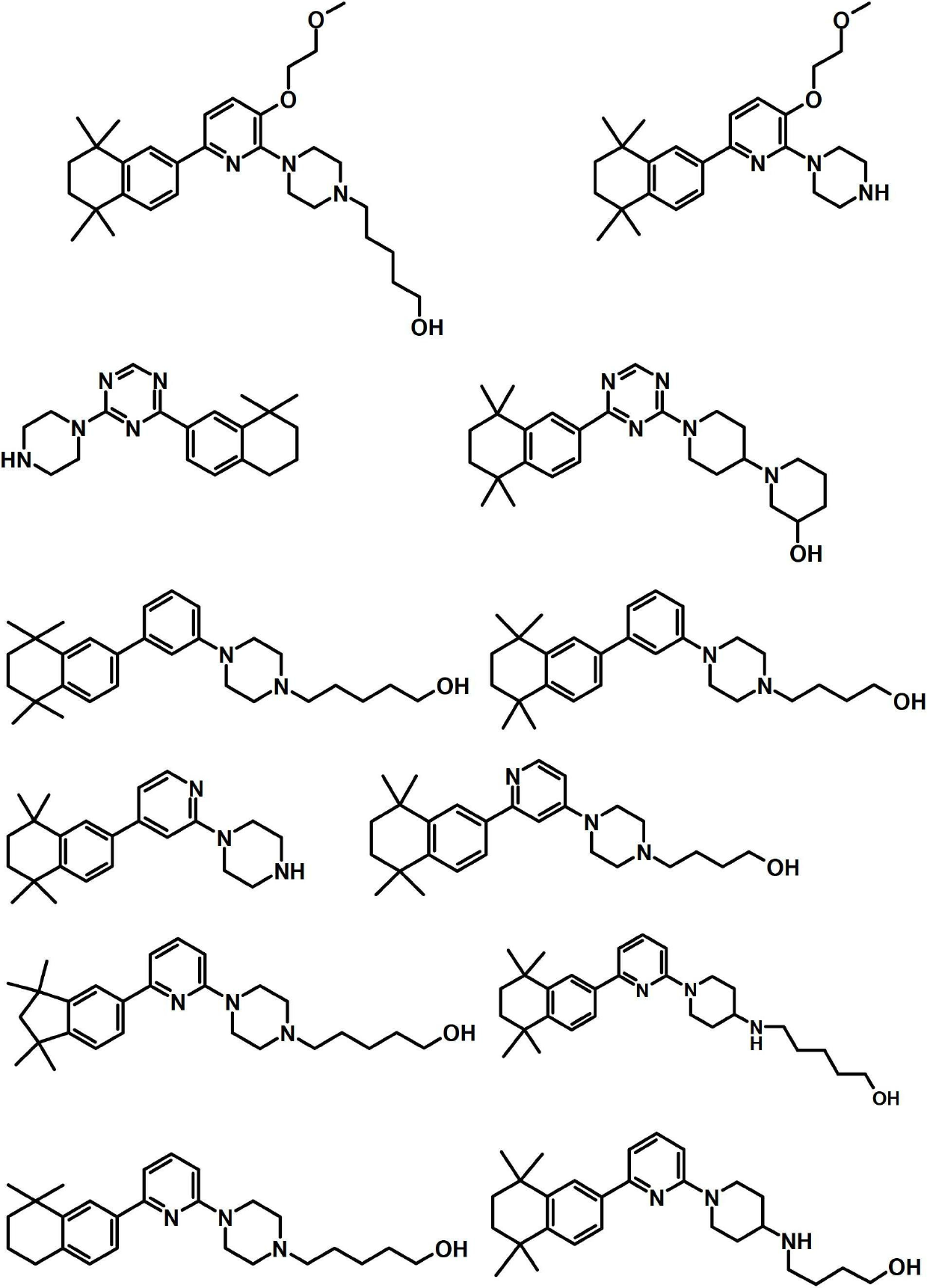
Sphingosine kinase inhibitors
A technology of solvates and compounds, applied in the field of sphingosine kinase inhibitors, can solve problems such as failure to identify interaction partners
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0330] I. Synthesis of Selected Compounds of the Invention
[0331] The following compounds were synthesized and identified. However, it is within the knowledge of those skilled in the art to prepare and identify these compounds in other ways.
[0332] FS101:
[0333] tert-butyl 4-[6-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)pyridin-2-yl]piperazine-1-carboxylate :
[0334]
[0335] 150 mg (0.44 mmol) of 4-(6-bromopyridin-2-yl) piperazine-1-carboxylic acid tert-butyl ester, 112 mg (0.48 mmol) of 5,5,8,8-tetramethyl-5, 6,7,8-tetrahydronaphthalen-2-ylboronic acid and 202 mg (0.88 mmol) of tripotassium phosphate monohydrate were suspended in 6 ml of ethylene glycol monomethyl ether, degassed several times, and degassed under a nitrogen atmosphere 25 mg (0.04 mmol) of bis(triphenylphosphine)palladium(II) dichloride were added. The reaction mixture was stirred at 80° C. for 16 hours, cooled to room temperature, and 20 ml of water were added. The mixture was ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com