Preparation method of flocoumafen
A technology of fluocorone and molar ratio, which is applied in the field of synthesis of the second-generation anticoagulant rodenticide, fluocorone, and can solve the problem of difficult to buy, irregular method, and conversion of methoxyphenyl benzyl ketone Low yield and other problems, to achieve the effect of simple and easy reaction, easy post-treatment operation, and increase the overall reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
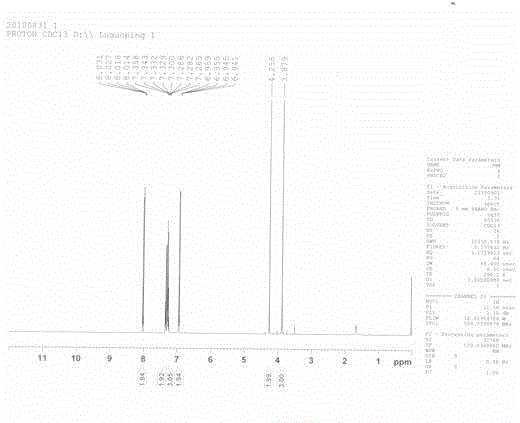
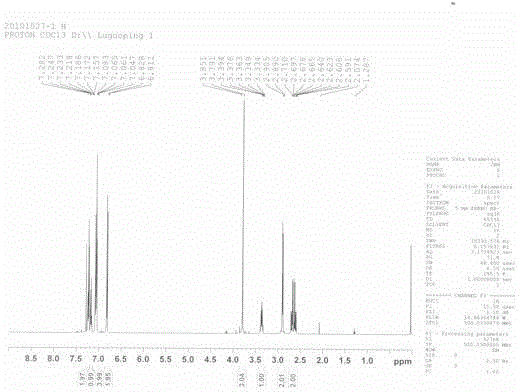
Image
Examples
Example Embodiment
[0047] Example 1
[0048] In the first step, 7.725g (0.05mol) of phenylacetyl chloride and 10.08g (0.10mol) of anisole were dissolved in 50mL of dichloromethane, 13.35g (0.10mol) of aluminum trichloride was added at -20°C, and stirred for 16h After the end, the reaction solution was poured into 10% hydrochloric acid, and the phases were separated. The organic phase was washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain the crude product, which was then recrystallized from methanol to obtain white crystals of 4-methoxybenzene. phenethyl ketone.
[0049] In the second step, 2.26g (0.01mol) of the product of the first step, 0.48g (0.02mol) of magnesium powder, and 1.67g (0.01mol) of ethyl bromoacetate were dissolved in 15mL of benzene, and 0.01 g of ethyl bromoacetate was added dropwise at 25°C under vigorous stirring. mol boron trifluoride·acetonitrile, and stir for 3-4h after the dropwise addition. After th...
Example Embodiment
[0185] Example 2
[0186] The reaction steps are exactly the same as in Example 1, except that:
[0187] For the first step, the anisole was changed to 5.04 g (0.05 mol) and aluminum trichloride was added at 15°C.
[0188] For the second step, 0.56g (0.01mol) iron powder was selected as the metal reagent, tetrahydrofuran was selected as the solvent, 6.68g (0.04mol) of ethyl bromoacetate was selected, and 0.01mol of boron trifluoride·tetrahydrofuran was added dropwise at 35°C.
[0189] For the third step, triethylsilane was changed to 3.48 g (0.03 mol), trifluoroacetic acid was changed to 1.14 g (0.01 mol), and 0.005 mol of boron trifluoride·tetrahydrofuran was used for the boron trifluoride complex.
[0190] For the fourth step, the amount of sodium hydroxide was changed to 3.20 g (0.08 mol), and the recrystallization reagent was methanol.
[0191] For the fifth step, the reaction temperature was 90° C., the amount of PPA was 16.90 g (0.05 mol), and the mixture was stirred...
Example Embodiment
[0197] Example 3
[0198] The reaction steps are exactly the same as in Example 1, except that:
[0199] For the first step, the anisole was changed to 7.56 g (0.075 mol), and 6.625 g (0.05 mol) of aluminum trichloride was added at 0°C.
[0200] For the second step, 2.56g (0.04mol) copper powder was selected as the metal reagent, toluene was selected as the solvent, and ethyl bromoacetate was changed to 3.34g (0.02mol), and 0.02mol boron trifluoride·ether was added dropwise at 55°C.
[0201] For the third step, triethylsilane was changed to 4.64 g (0.04 mol), trifluoroacetic acid was changed to 3.42 g (0.03 mol), and 0.02 mol of boron trifluoride·acetonitrile was used for the boron trifluoride complex.
[0202] For the fourth step, the amount of sodium hydroxide was changed to 1.60 g (0.04 mol), and acetonitrile was selected as the recrystallization reagent.
[0203] For the fifth step, the reaction temperature was 120° C., the amount of PPA was 10.14 g (0.03 mol), and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com