Construction method of recombinant HEK (human embryonic kidney) 293 cells highly expressing H1R (histamine receptor 1)
A construction method and high expression technology are applied in the field of recombinant HEK293 cell construction to achieve the effect of improving specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be described in detail below in conjunction with the accompanying drawings and embodiments.
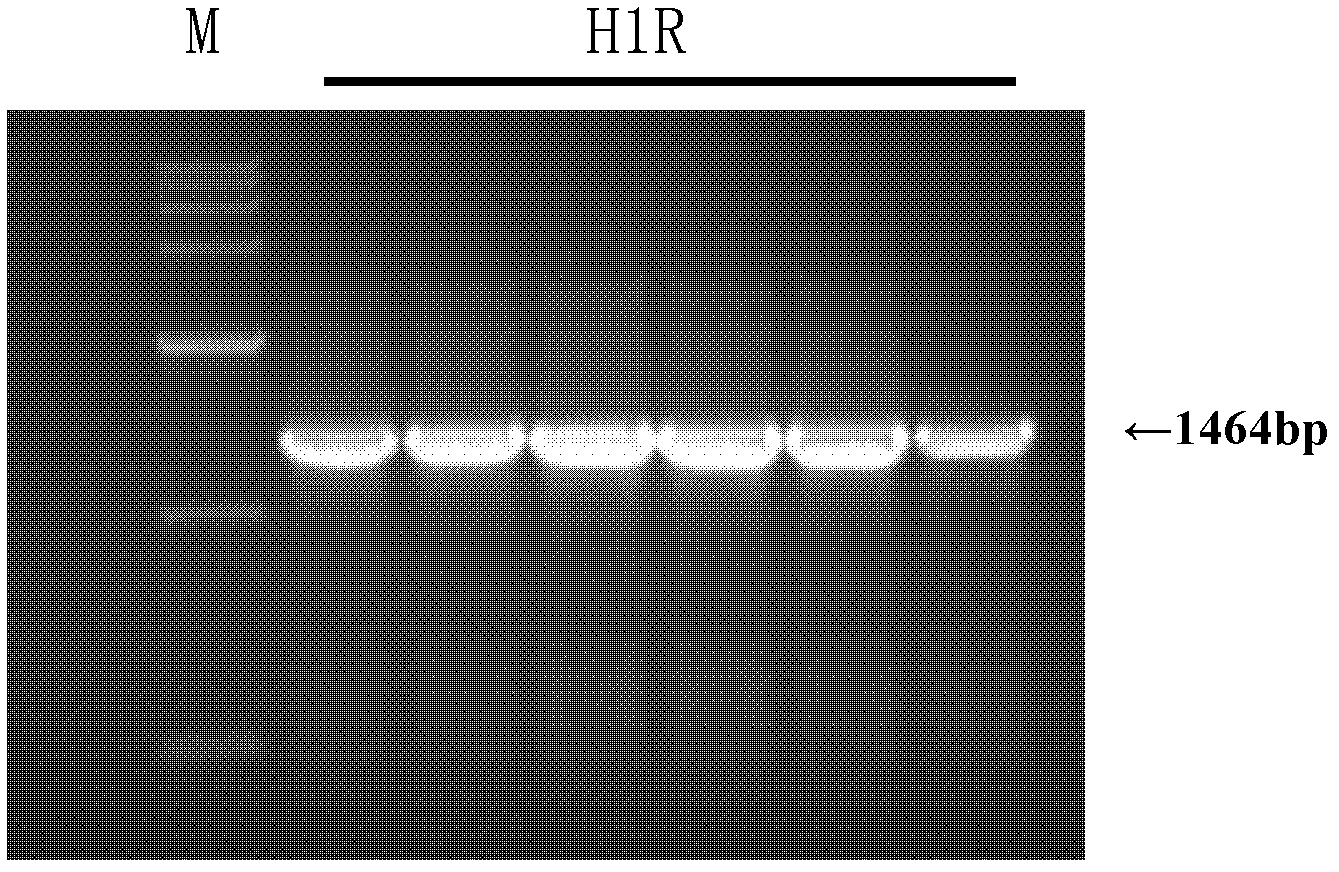
[0025] In the present invention, on the basis of constructing the eukaryotic expression vector pEGFP-N1 / H1R of the full-length H1R gene, HEK293 cells are transfected to obtain a recombinant HEK293 / H1R cell line that is stable and highly expresses H1R. The following is an explanation of the embodiments of the present invention. Not limited.
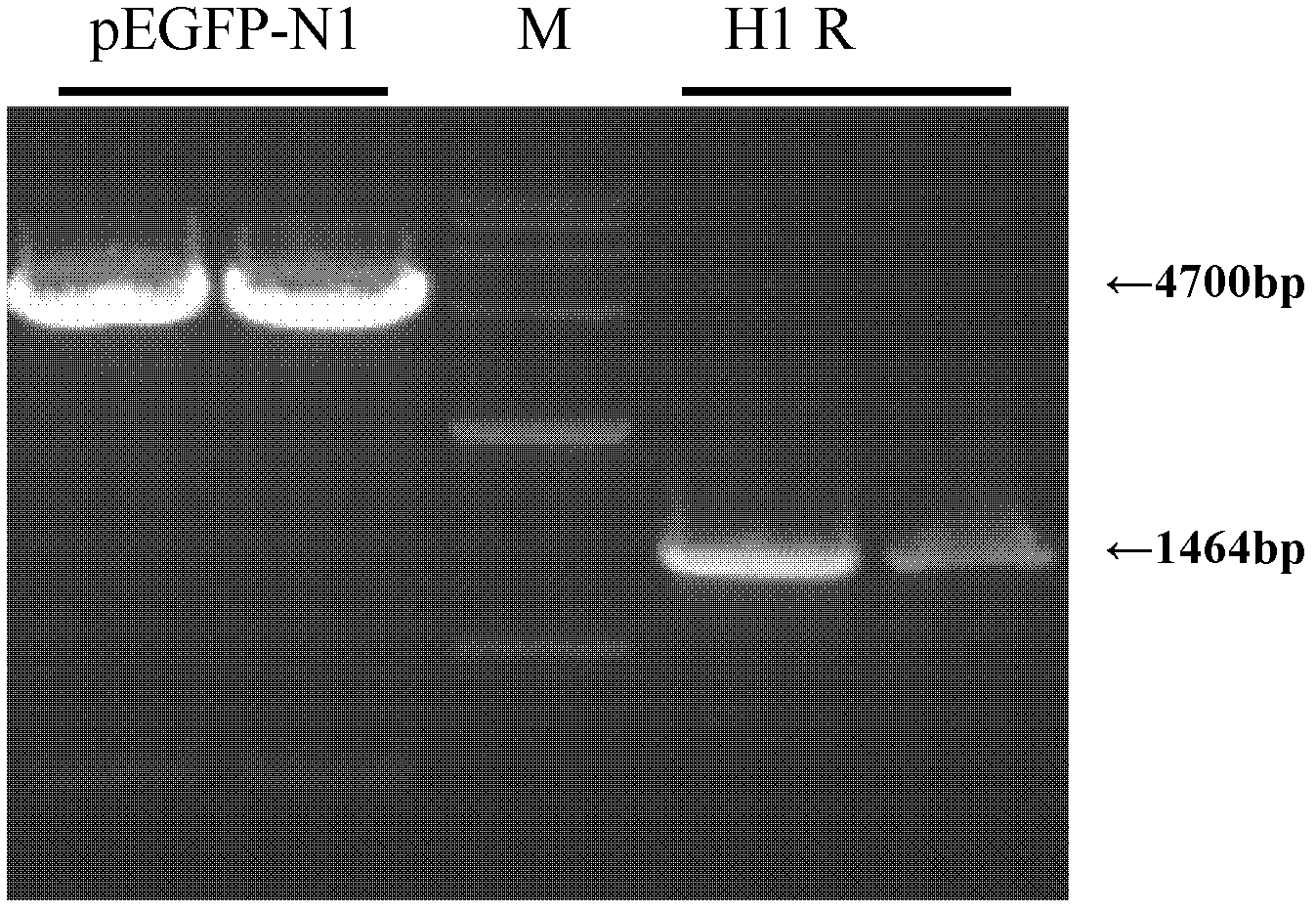
[0026] In this example, pEGFP-N1 was used as the base vector, and HindIII and XhoI restriction sites were specifically selected as multiple cloning sites for connecting foreign genes.
[0027] A method for constructing recombinant HEK293 cells with high H1R expression, comprising the following steps:
[0029] Select the pBluescriptR / H1 plasmid containing the full-length cDNA sequence of the H1R gene as a template for cloning, use Primer Premier5.0 software to design corresponding spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com