Polybenzoxazine composition
A technology of polymer composition, benzoxazine, applied in coating, chemical recycling, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0108] All parts, percentages, ratios, etc. in these examples are by weight unless otherwise indicated. Solvents and other reagents used were obtained from Sigma-Aldrich Chemical Company (Milwaukee, WI) unless otherwise indicated.
[0109] Material
[0110] Benzo Azine A: XU3560 TM Benzo Oxyzine is bis(3-phenyl-3,4-dihydro-2H,3-benzo azinyl) isopropane, a bisphenol-derived benzo Zine, available from Huntsman Corporation, The Woodlands TX.
[0111] JEFFAMINES TM D400 and D2000 are diamine-terminated poly(oxyalkylenes) with molecular weights of about 400 and 2000, respectively. All JEFFAMINEs were obtained from Huntsman Corporation.
[0112] Benzo Zine B * is using benzo Azine B (the following is based on JEFFAMINE TM D400 Benzo oxazine) and compounded with 25% by weight of silicone-based core-shell particles from Kaneka Texas Corporation (Pasadena, TX).
[0113] Benzo Azine A * is using benzo Zine A (60 wt %), MEK (20 wt %) and core-shell particles...
preparation example
[0126] Benzo Zine B and C
[0127] By mixing Jeffamine D-400 in a 2L round bottom flask equipped with a reflux condenser TM A mixture of diamine (43 g, 0.1 mol), paraformaldehyde (13.2 g, 0.44 mol) and phenol (18.8 g, 0.2 mol) to prepare benzos derived from poly(ethylene oxide) diamine Zine B. The mixture was then heated to 100°C for 10 hours. The reaction mixture was cooled and condensed water was removed under reduced pressure. The resulting product (ca. 95% yield, structure confirmed by NMR) was used without further purification.
[0128] Using the same procedure, using the diamine JEFFAMINE TM D2000 Preparation of Benzo Zinc C.
[0129] DEG-SbF 5 acid catalyst
[0130] The catalyst was prepared according to the procedure disclosed in US Patent 4,503,211 (Robins) but without the use of hindered amines. Representative preparations are as follows:
[0131] In a 100 mL 3-neck flask equipped with a stirrer, addition funnel, thermometer and means to excl...
example 1-13
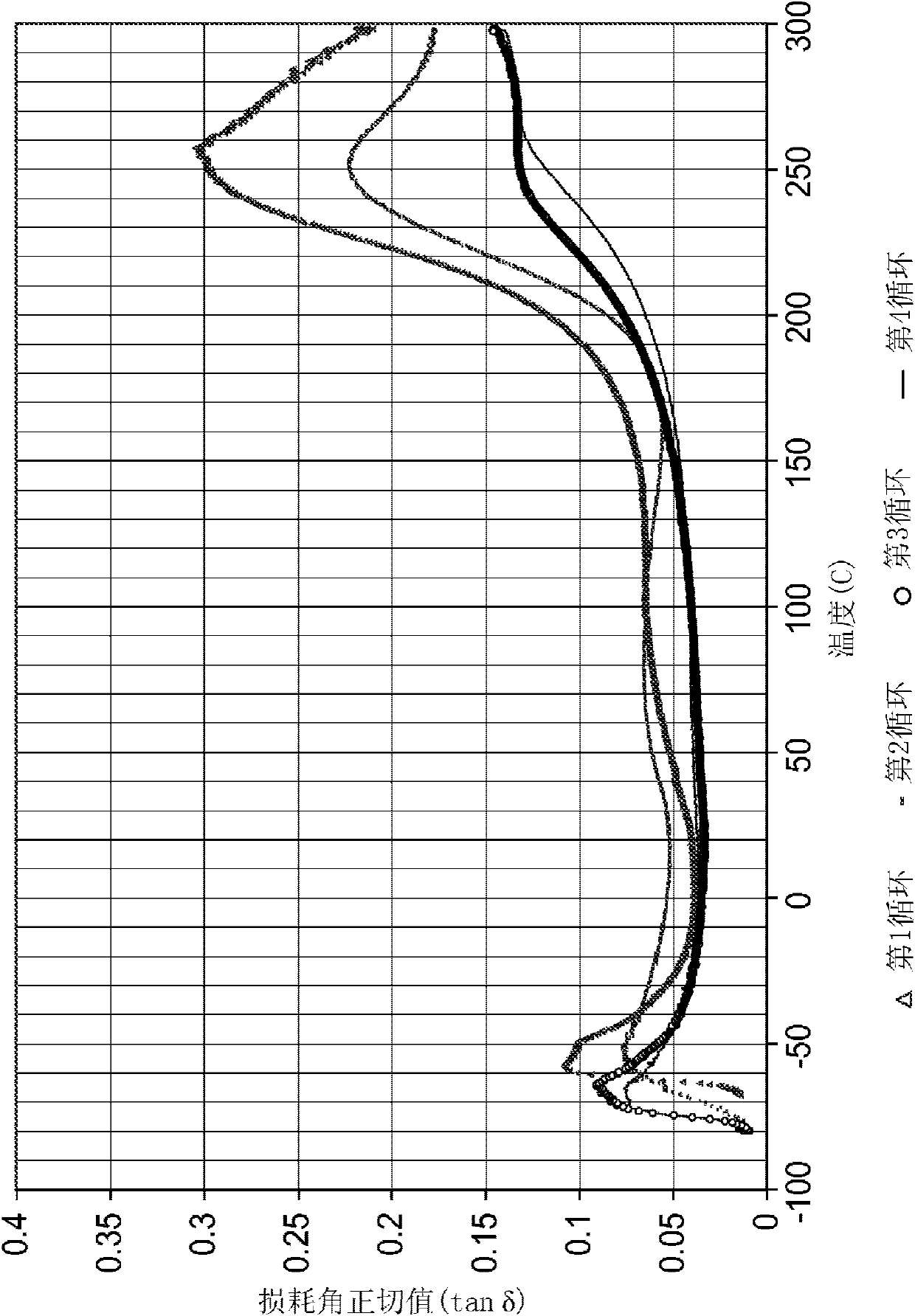
[0133] The benzos mixed in the specified ratio The oxazine was heated to 130°C in a metal container and held for 30 minutes. Stir the mixture, let it cool to about 100°C, and add 5% DEG*SbF to it 5 catalyst. The resulting solution was then cast into a silicone mold placed between two silicone release liner coated PET sheets. The mold consisted of an approximately 1 mm thick sheet with rectangular cutouts (approximately 5 mm wide x 30 mm long) and square cutouts (approximately 10 mm x 10 mm) to prepare samples for dynamic mechanical analysis.
[0134] The assembly was then sandwiched between two glass sheets and allowed to cure at 177°C for 30 minutes. The gripper assembly was then cooled to room temperature before the samples were removed and evaluated using a Seiko Instruments Dynamic Mechanical Analyzer (DMA) in tensile mode at temperatures ranging from -80°C to 300°C. Cured samples were clear and dark amber in color. After the manipulation, the samples were removed, i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com