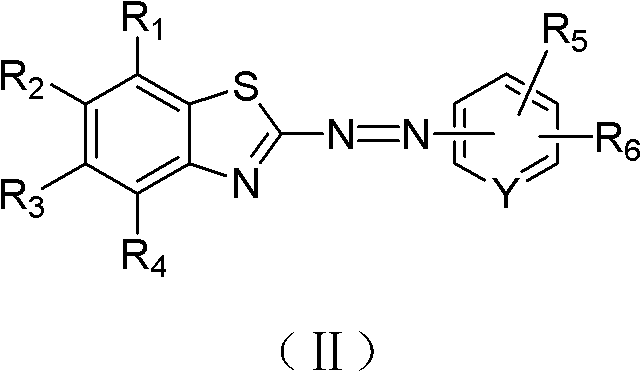
Benzothiazole derivative and application thereof
A technology of benzothiazole derivatives, which is applied in the field of benzothiazole derivatives, can solve the problems of slow brain clearance, postmortem diagnosis, large trauma and risk of biopsy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the synthetic method of benzothiazole Schiff base compound
[0052]
[0053] The synthesis of benzothiazole Schiff base compounds is formed by condensation of 2-aminobenzothiazole and substituted benzaldehyde, refluxed in ethanol, organic acid (such as acetic acid, p-toluenesulfonic acid) or Lewis acid (such as chlorine ZnC) catalysis.
Embodiment 2
[0054] Embodiment 2: the synthetic method of benzothiazole azo compound
[0055]
[0056] The synthesis of benzothiazole azo compounds firstly reacts 2-aminobenzothiazole and sodium nitrite at low temperature under acidic conditions to form diazonium salts, and then couples with aromatic compounds to obtain benzothiazole azo compounds.
Embodiment 3
[0057] Embodiment 3: benzothiazole derivatives 11 C radioisotope labeling method
[0058]
[0059] Benzothiazole derivatives 11 C radioisotope labeling includes the amino and hydroxyl groups on the aromatic ring 11 C radioactively labeled. Among them, the radioisotope modification of amino and methylamino can be obtained 11 C-labeled methylamino and dimethylamino; radioactive isotope modification of the phenolic hydroxyl group can be obtained 11 C labeled methoxy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com