Method for preparing 2-aldehyde oxazole
A technology of aldooxazole and oxazole, which is applied in the field of preparation of 2-aldooxazole, can solve the problems of unsuitable storage and transportation, cumbersome post-processing, and low yield, and achieve convenient storage and transportation, and simple post-processing methods , The effect of stable process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
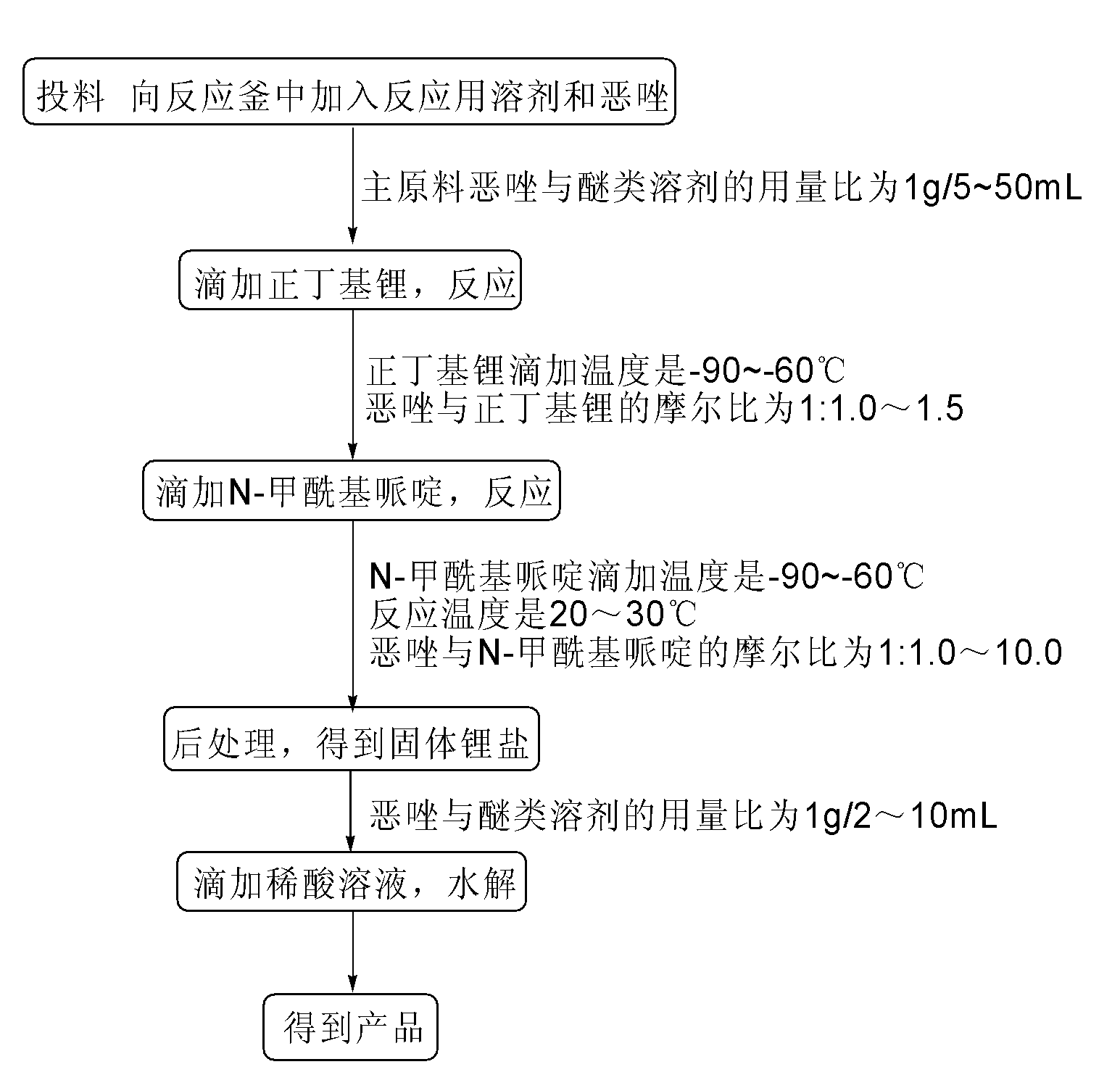
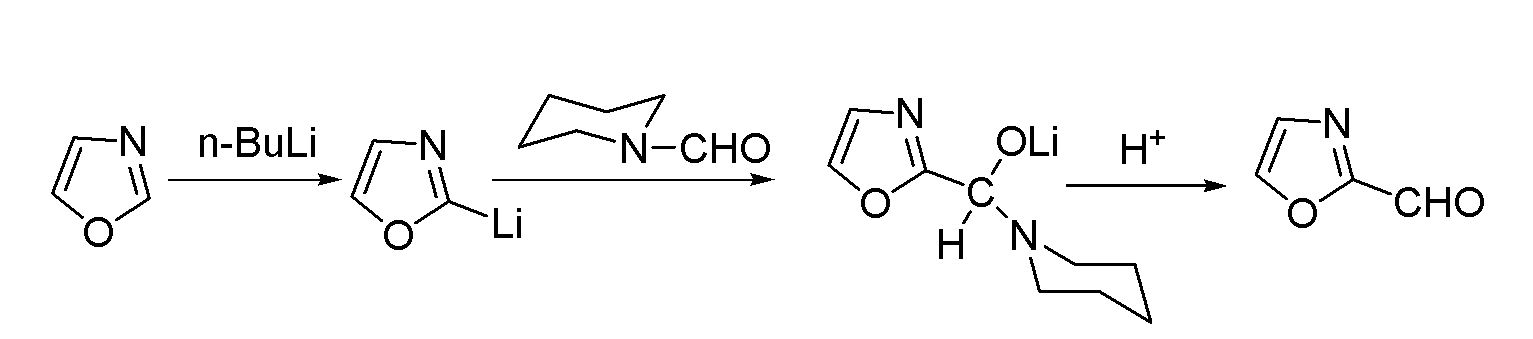
[0025] Embodiment 1: a kind of method for preparing 2-formyl oxazole is characterized in that concrete steps are as follows:
[0026] (1) Feeding: Add 20.0kg of main raw material oxazole into the 1500L reactor 600L tetrahydrofuran, start stirring;
[0027] (2) Add n-butyllithium solution dropwise: cool the system down to -65±5°C, and add 24.1kg of n-butyllithium solution dropwise at -65±5°C, after dropping, keep warm at -65±5°C 5h;
[0028] (3) Add N-formylpiperidine dropwise: Add 163.8kg N-formylpiperidine dropwise to the system at -65±5°C, after the drop is complete, the system is warmed to 25±5°C and kept at 20±5°C for 50 hours ;
[0029] (4) Post-treatment: Cool the system to 0±3°C, stir and crystallize, filter with suction, rinse the filter cake with 80L methyl tert-butyl ether, and dry it under nitrogen protection to obtain solid lithium salt NMR internal standard purity (NMR): 98.8%, yield 85.0%;
[0030] (5) Hydrolysis: To the solid lithium salt obtained in step...
Embodiment 2
[0032] Embodiment 2: a kind of method for preparing 2-formyl oxazole is characterized in that concrete steps are as follows:
[0033] (1) Feeding: Add 6.5kg of the main raw material oxazole and 227.5L of methyl tert-butyl ether into the 500L reactor, and start stirring;
[0034] (2) Add n-butyllithium solution dropwise: cool the system to -85±5°C, and add 7.2kg of n-butyllithium solution dropwise at -85±5°C, after dropping, keep warm at -85±5°C 5h;
[0035] (3) Adding N-formylpiperidine dropwise: Add 106.5kg N-formylpiperidine dropwise to the system at -85±5°C, after the drop is complete, the system is warmed to 25±5°C and kept at 25±5°C for 48 hours ;
[0036] (4) Post-treatment: Cool the system to 0±3°C, stir and crystallize, filter with suction, rinse the filter cake with 80L methyl tert-butyl ether, and dry it under nitrogen protection to obtain solid lithium salt NMR internal standard purity (NMR): 98.2%, yield 80.5%;
[0037] (5) Hydrolysis: Add dropwise 65L of dilu...
Embodiment 3
[0038] Embodiment 3: a kind of method for preparing 2-formyl oxazole is characterized in that concrete steps are as follows:
[0039] (1) Feeding: Add 2.8kg of main raw materials oxazole and 20L tetrahydrofuran into a 100L reaction bottle, and start stirring;
[0040](2) Add n-butyllithium solution dropwise: cool the system down to -65±5°C, and add 2.8kg of n-butyllithium solution dropwise at -65±5°C, after dropping, keep warm at -65±5°C 2h;
[0041] (3) Adding N-formylpiperidine dropwise: Add 4.6kg N-formylpiperidine dropwise to the system at -65±5°C, after the drop is complete, the system is warmed to 25±5°C and kept at 25±5°C for 32 hours ;
[0042] (4) Post-treatment: Cool the system to 0±3°C, stir and crystallize, filter with suction, rinse the filter cake with 2.8L methyl tert-butyl ether, and dry it under nitrogen protection to obtain solid lithium salt. (NMR): 98.5%, yield 75.0%;
[0043] (5) Hydrolysis: Add 5.6L of 20% dilute acetic acid solution dropwise to the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com