Application of EDTA (ethylene diamine tetraacetic acid) in improving exocytosis volume and expression volume of escherichia coli recombinant protein
A technology of Escherichia coli and extracellular secretion, which is applied in the biological field and can solve the problems of low expression of exogenous recombinant proteins, inactive inclusion bodies, and inability to secrete
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Materials
[0048] 1. Strains and plasmids
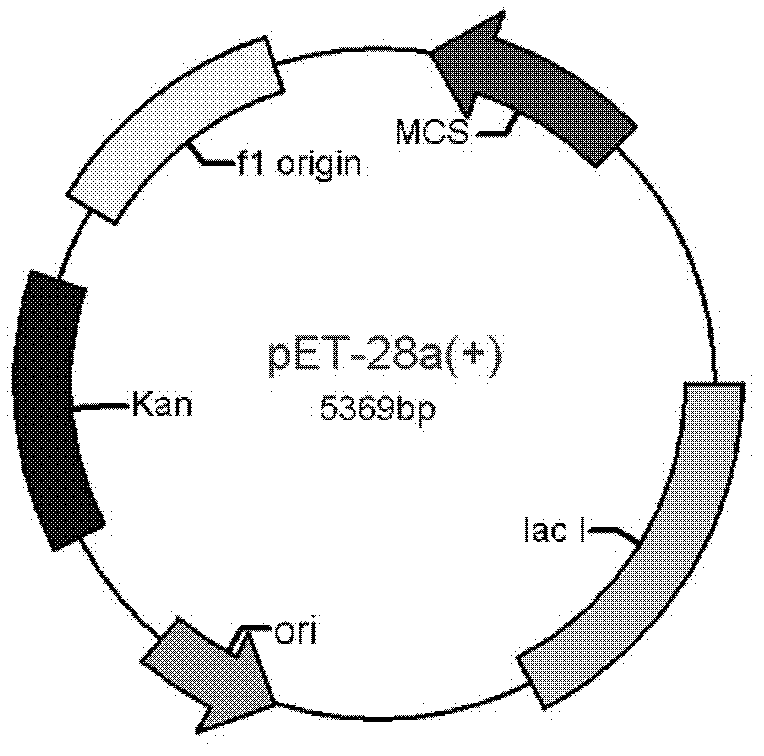
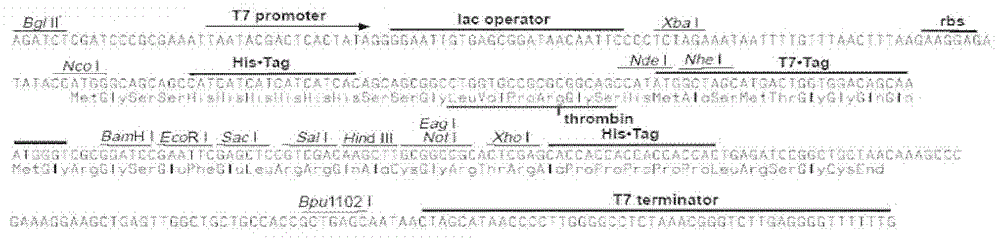
[0049] Bacillus sp.III-3 (preservation number: CGMCC No.2138, preservation date: August 22, 2007, patent number: 200710030787.6, name: an alkalophilic bacillus and the endoglucan produced by it Carbohydrase and Application) is used as the original bacteria of the alkaline cellulase gene, E.coli Top10 (Invitrogen) is used for the maintenance and operation of the plasmid, and E.coli BL21Star (DE3) (Invitrogen) is used as the expression host bacteria. The expression plasmid is pET-28a (+) (Invitrogen company, its plasmid map is as follows figure 1 As shown, the map of multiple cloning sites is shown in figure 2 shown).
[0050] 2. Medium
[0051] Each liter medium contains: 10g compound peptone, 5g yeast powder, 5NaCl, pH value is 7.
[0052] 3. Instrument
[0053] MyCyclerTM thermal cycler PCR instrument, DNA and protein electrophoresis system are products of Bio-rad; spectrophotometer and micro-spectrophotometer are p...
Embodiment 2
[0105] (1) Experimental method
[0106] 1. Construction of recombinant Escherichia coli
[0107] Bacillus subtills.32356 (purchased from China Industrial Microorganism Culture Collection Management Center, preservation number: CICC No.20636, as the original bacterium of amylase gene) genome acquisition method refers to the instructions of Omega gene extraction kit; DNA restriction enzyme Cutting, ligation, and PCR amplification all refer to the instructions of Takera reagents; the primers (Shanghai Sangong Synthetic) used to amplify the amylase gene are:
[0108] Upstream primer (Nde I): 5′-GTC CATATG GTAAATGGCACGCTGATG-3′;
[0109] Downstream primer (BamH I): 5′-CGC GGATCC TTATTTCTGAACATAAATGGAGAC-3';
[0110] The PCR product and the expression plasmid pET-28a(+) were ligated and transformed into E.coli Top10 after double enzyme digestion to obtain the recombinant plasmid pET-28a-A, transformed into E.coli BL21Star(DE3), and subjected to liquid PCR and sequencing (nucle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com