Method for synchronous determination of content of triazole chiral pesticide enantiomers
A technology for enantiomers and triazoles, which is applied in the field of simultaneous determination of the enantiomer content of triazoles chiral pesticides, can solve the problem of the inability to achieve simultaneous separation of multiple chiral pesticide enantiomers, high-sensitivity detection, and influence methods. selectivity and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
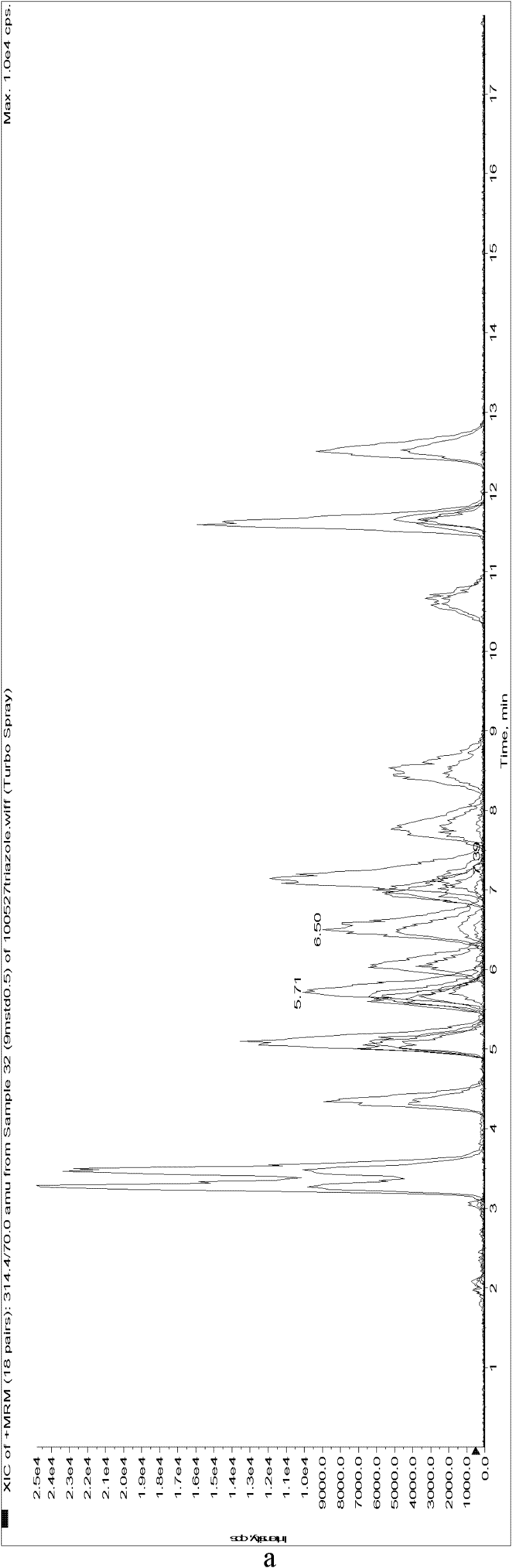
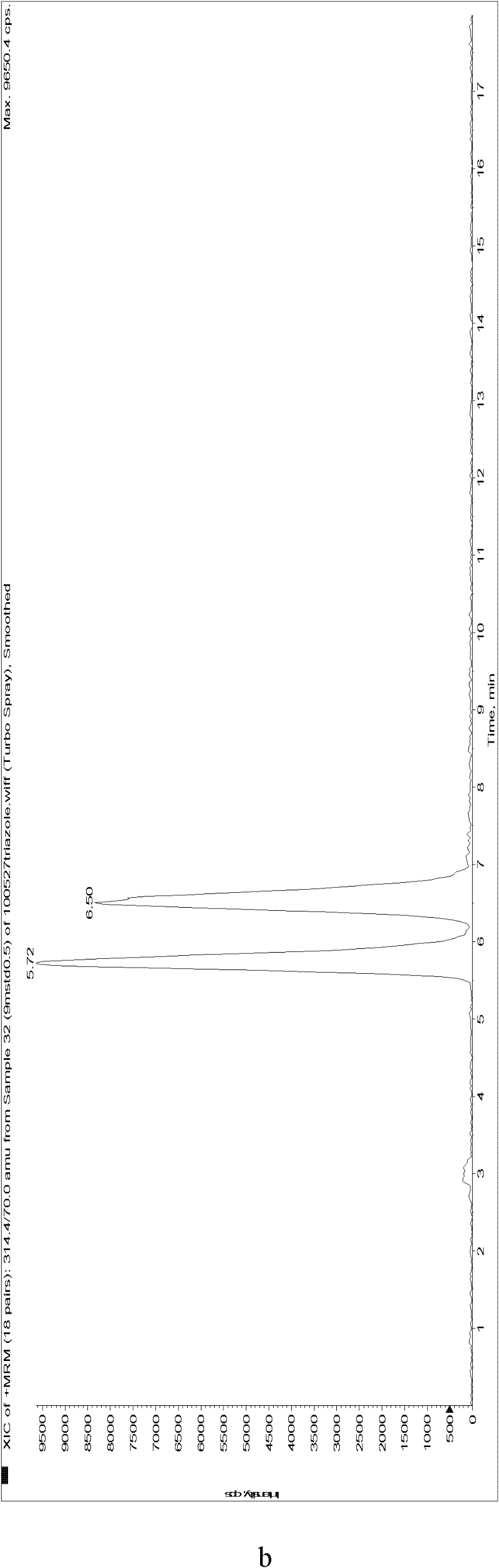
Image
Examples
Embodiment 1
[0104] Example 1, Characteristic Separation of Enantiomers of Triazole Chiral Pesticides
[0105] 1. Investigation of Enantiomeric Separation Conditions
[0106] A Phenomenex Lux Cellulose-1 chiral chromatography column packed with cellulose-3,5-dimethylphenylcarbamate chiral stationary phase with a particle size of 3 μm was used, and acetonitrile / water and methanol / water were used as mobile phases In reversed-phase liquid chromatography, the enantiomers of the above nine chiral pesticides were resolved with a flow rate of 0.3mL / min and a detection wavelength of 220nm, and the volume ratios of acetonitrile / water were 90 / 10, 80 / 20 , 70 / 30, 60 / 40, 50 / 50, and methanol / water volume ratios of 90 / 10, 85 / 15, 80 / 20, 75 / 25, 70 / 30, respectively, the impact on enantiomeric resolution, the results See Table 4 and Table 5.
[0107] Table 4. Chromatographic separation parameters of enantiomers of triazole chiral pesticides
[0108]
[0109] Table 5, cyproconazole enantiomeric chromato...
Embodiment 2、 3
[0128] Embodiment 2, synchronous determination of triazole chiral pesticide enantiomer content
[0129] 1. Synchronous separation of enantiomers and establishment of LC / MS / MS analysis method
[0130] The standard solutions of 9 kinds of chiral pesticides were used to optimize the mass spectrometry parameters on the API2000 triple quadrupole tandem mass spectrometer, including the selected multiple reaction monitoring (MRM) quantitative and qualitative ion pairs, collision energy (CE), solution Cluster voltage (DP), etc., the optimization results are shown in Table 2.
[0131] Table 2. Mass Spectrometry Detection Conditions
[0132]
[0133] Under these conditions, it was combined with liquid chromatography, and the chromatographic separation conditions of 9 chiral pesticide enantiomers were optimized by using acetonitrile / water constant ratio and gradient elution procedures. For better enantiomeric separation and chromatographic peak shape, the optimal elution conditions ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com