Amino thiazole derivative and preparation method and medical purpose thereof
A technology of aminothiazole and derivatives, applied in the field of medicine, can solve problems such as difficult to cure neurodegenerative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
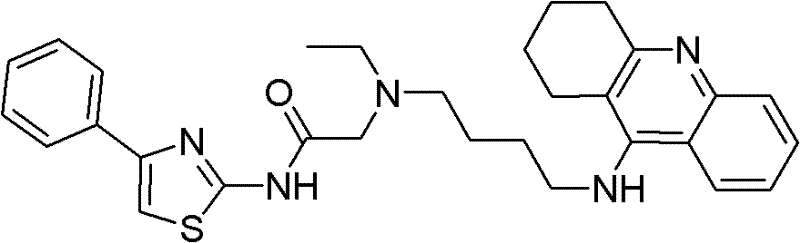
[0042] 2-(Ethyl(4-(1,2,3,4-tetrahydro-acridin-9-amino)butyl)amino)-N-(4-phenylthiazol-2-yl)acetamide preparation of
[0043]
[0044] Reagents: 2-bromo-N-(4-phenylthiazol-2-yl)acetamide (0.4 g, 1.57 mmol), DMF (10 mL), potassium carbonate (0.26 g), potassium iodide (0.136 g), and N- (4-(Ethylamino)butyl)-1,2,3,4-tetrahydroacridin-9-amine (0.5 g, 1.57 mmol). Method: Take 2-chloro-N-(4-phenylthiazol-2-yl)acetamide and N-(4-(ethylamino)butyl)-1,2,3,4-tetrahydroacridine- 9-amine was dissolved in N,N-dimethylformamide, potassium carbonate was added, and the temperature was controlled at 70°C for 2 hours. Diluted with dichloromethane, washed with water and dried, then directly purified by silica gel column chromatography. Purification method: silica gel column chromatography using petroleum ether / ethyl acetate (2:1, 0.3% triethylamine) to obtain 0.51 g of light yellow oil with a yield of 55%. h 1 NMR results are: H 1 -NMR (CDCl 3 , 400MHz, δppm): 7.92(d, J=7.6Hz, 1H), 7.91...
Embodiment 1-1
[0046] N-(4-(4-chlorophenyl)thiazol-2-yl)-2-(ethyl(4-(1,2,3,4-tetrahydro-acridin-9-amino)butyl) Preparation of amino)acetamide
[0047]
[0048] Reagents: 2-bromo-N-((4-chlorophenyl)thiazol-2-yl)acetamide (0.34 g, 117 mmol), DMF (10 mL), potassium carbonate (0.2 g), potassium iodide (0.05 g), and N-(4-(Ethylamino)butyl)-1,2,3,4-tetrahydroacridin-9-amine (0.35 g, 1.17 mmol). Except for the reagents, the rest of the preparation and purification methods were the same as in Example 1 to obtain 0.6 g of a light brown oil with a yield of 85.7%. h 1 NMR results are: H 1 -NMR (CDCl 3 , 400MHz, δppm): 7.90(d, J=7.6Hz, 1H), 7.88(d, J=7.6Hz, 1H), 7.74(d, J=8.4Hz, 2H), 7.52(m, 2H), 7.33 (d, J=8.4Hz, 2H), 7.29(m, 1H), 7.11(s, 1H), 3.47(t, J=7.2Hz, 2H), 3.27(s, 2H), 3.03(t, J= 6.0Hz, 2H), 2.66(m, 4H), 2.59(m, 2H), 1.85(m, 4H), 1.69(m, 2H), 1.59(m, 2H), 1.08(t, J=7.2Hz, 3H).C 13 NMR results are: C 13 -NMR (CDCl 3 , 100MHz, δppm): 170.1, 158.3, 157.2, 150.5, 148.9, 147.2, 133.7, ...
Embodiment 1-2
[0050] N-(4-(4-methoxyphenyl)thiazol-2-yl)-2-(ethyl(4-(1,2,3,4-tetrahydro-acridin-9-amino)butyl base) amino) acetamide preparation
[0051]
[0052] Reagents: 2-bromo-N-((4-methoxyphenyl)thiazol-2-yl)acetamide (0.43g, 1.5mmol), DMF (10mL), potassium carbonate (0.22g), potassium iodide (0.01h ), and N-(4-(ethylamino)butyl)-1,2,3,4-tetrahydroacridin-9-amine (0.45 g, 1.5 mmol). Except for the reagents, the rest of the preparation and purification methods were the same as in Example 1 to obtain 0.35 g of a light brown oil with a yield of 43.8%. h 1 NMR results are: H 1 -NMR (CDCl 3 , 400MHz, δppm): 7.95(d, J=7.6Hz, 1H), 7.93(d, J=7.6Hz, 1H), 7.75(d, J=8.8Hz, 2H), 7.55(m, 1H), 7.34 (m, 1H), 7.02(s, 1H), 6.92(d, 2H), 3.84(s, 3H), 3.52(t, J=7.2Hz, 2H), 3.29(s, 2H), 3.07(t, J=6.0Hz, 2H), 2.68(m, 4H), 2.61(t, J=7.2Hz, 2H), 1.88(m, 4H), 1.74(m, 2H), 1.62(m, 2H), 1.11( t,J=7.2Hz,3H).C 13 NMR results are: C 13 -NMR (CDCl 3 , 100MHz, δppm): 170.0, 159.5, 158.2, 156.9, 150.7, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com