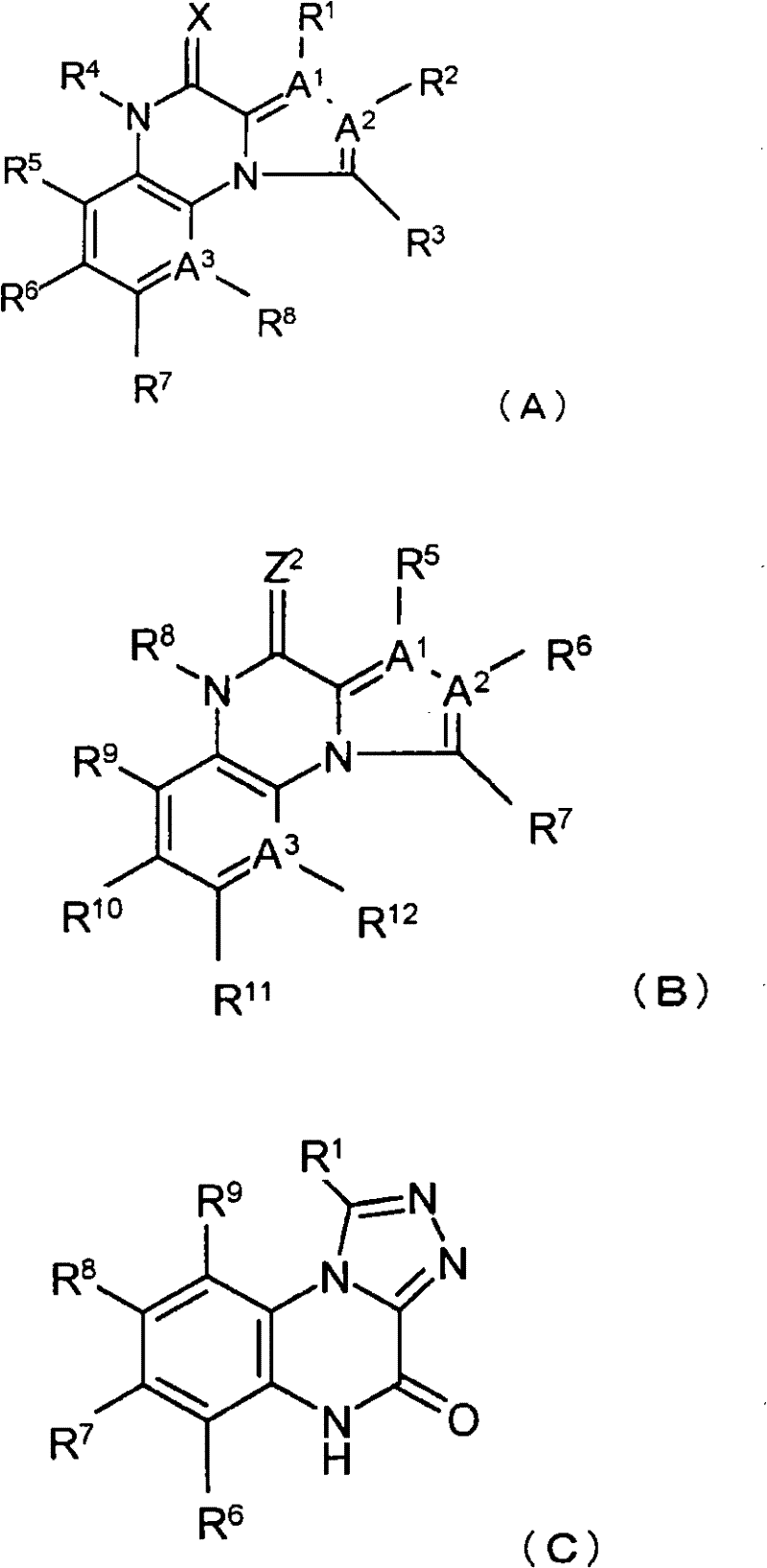
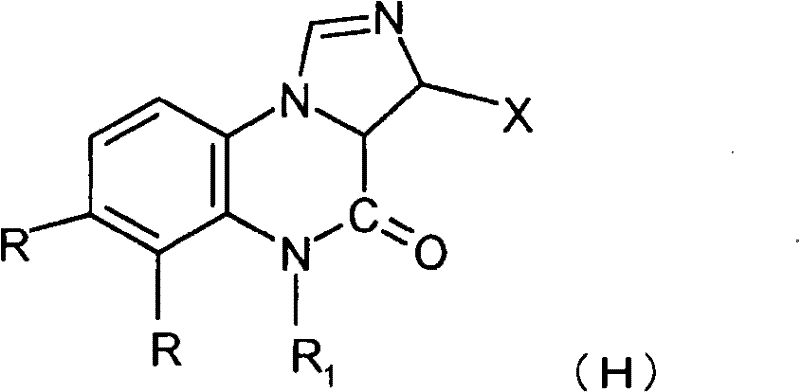
Quinoxaline compounds
A compound and quinoxaline technology, applied in the field of quinoxaline compounds, can solve the problems of no specific disclosed compound, unrecorded compound having PDE9 inhibitory effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0285] Embodiment 3 (6.7), embodiment 4 (19), embodiment 5 (2.3), embodiment 11 (3.1), embodiment 13 (22), embodiment 27 (18), embodiment 35 (12), implement Example 68 (3.5), Example 71 (9.2), Example 76 (2.5), Example 77 (1.0), Example 80 (2.6), Example 91 (20), Example 93 (15), Example 103(11), Example 134(950), Example 220(18), Example 248(5.8), Example 263(0.9), Example 277(16), Example 279(10), Example 281 (1.6), Example 289 (3.9), Example 290 (1.0), Example 291 (2.8), Example 300 (0.8), Example 306 (4.2), Example 309 (4.1), Example 312 ( 20), Example 318 (5.9), Example 320 (1.0), Example 322 (29), Example 324 (1.7), Example 326 (1.2), Example 330 (1.2), Example 333 (2.9 ), Example 338 (28), Example 343 (13), Example 344 (7.7), Example 346 (27), Example 347 (3.3), Example 350 (9.2), Example 352 (12) , Example 356 (1.5), Example 359 (5.4), Example 516 (0.8), Example 517 (0.9), Example 519 (6.8), Example 522 (3.6), Example 528 (0.9).
[0286] In addition, among the compo...
Embodiment
[0308] Hereinafter, the production method of the compound of formula (I) is demonstrated in more detail based on an Example. In addition, the present invention is not limited to the compounds described in the following Examples. In addition, the production methods of the raw material compounds are described in the production examples, respectively. In addition, the production method of the compound of formula (I) is not limited to the production method of the specific examples shown below, and the compound of formula (I) can also be produced by a combination of these production methods or methods obvious to those skilled in the art. manufacture.
[0309] In Examples, Production Examples, and Tables described later, the following abbreviations may be used. tert-: tertiary, Pr: Production example number, Ex: Example number, No: Compound number, Structure: Structural formula, Syn: Production method (the number indicates that the example compound is produced by the same producti...
manufacture example 1
[0311] Suspend 700 mg of methyl 3-chloro-2-hydrazinoquinoxaline-6-carboxylate in 3.7 mL of trimethyl orthobutyrate, and heat to reflux for 2 hours. The solid was filtered off after ice cooling and washed with cold ethyl acetate. The obtained solid was washed with hot ethyl acetate to obtain 612 mg of methyl 4-chloro-1-propyl[1,2,4]triazolo[4,3-a]quinoxaline-7-carboxylate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com