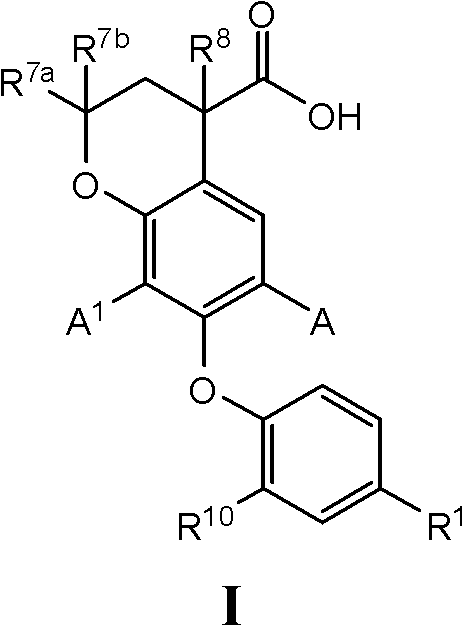
7-phenoxychroman carboxylic acid derivatives
A technology of phenyl and alkyl, applied in the field of 7-phenoxychroman carboxylic acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0524] DP-2 binding inhibition assay
[0525] The coding sequence for human DP2 was introduced into the human leukemia cell line K562 by electroporation and stable clones expressing DP2 were obtained by limiting dilution followed by staining of the cell surface with a rat monoclonal antibody specific for human DP2. Membranes were prepared from one of these DP2 expressing clones and used to determine the ability of compounds of the invention to inhibit the binding of prostaglandin D2 (PGD2) to its receptor DP2 by the following procedure in the presence of one or more of the following serum protein concentrations: Concentrations were 0.1% BSA, 1% HSA or 4% HSA. Cell membranes (1.25 μg / well for 0.1% BSA and 6 μg / well for 1% or 4% HSA) were mixed with the 3 H-labeled PGD 2 and various concentrations of test compounds in 150 microliters of binding buffer (50mM Tris-HCl (pH7.4), 40mM MgCl 2 , 0.1% fetal bovine serum albumin, 0.1% NaN 3 ) mixed in. After 60 min incubation at roo...
Embodiment 1
[0553] 7-(4-((5-(trifluoromethyl)pyridin-2-yl)carbamoyl)phenoxy)-6-cyanochroman-4-carboxylic acid
[0554]
[0555] Step A: Preparation of 7-fluoro-4-(trimethylsilyloxy)chroman-4-carbonitrile: 7-fluoro-2,3-dihydrochromen-4-one (470 mg, 2.829 mmol) and ZnI 2 (45.15 mg, 0.1414 mmol) was diluted with trimethylsilyl cyanide (1.413 mL, 11.32 mmol). The reaction mixture was stirred at ambient temperature for 4 hours. The reaction mixture was diluted with dichloromethane and washed with saturated sodium bicarbonate. The organic layer was dried over magnesium sulfate, filtered and concentrated to afford the title compound (750 mg, 99.92% yield).
[0556] Step B: Preparation of 7-fluoro-3,4-dihydro-2H-chromene-4-carboxylic acid: 7-fluoro-4-(trimethylsilyloxy)chroman-4-carbonitrile (750 mg, 2.83 mmol) and SnCl 2 The dihydrate (2551 mg, 11.3 mmol) was diluted with glacial acetic acid (3 mL) and cone. HCl (3 mL). The reaction mixture was heated at 130 °C overnight in an oil bath....
Embodiment 2
[0564] 7-(4-(5-Chloropyridin-2-ylcarbamoyl)phenoxy)-6-cyanochroman-4-carboxylic acid
[0565]
[0566] Step A: Preparation of 6-cyano-7-fluoro-3,4-dihydro-2H-chromene-4-carboxylic acid: At ambient temperature, 6-cyano-7-fluoro-3,4-di Hydrogen-2H-chromene-4-carboxylate methyl ester (3.3 g, 14.0 mmol) and LiOH-H 2 O (5.89 g, 140 mmol) was stirred together in THF (25 mL) and water (25 mL) for 1 h. The reaction mixture was diluted with ether and water and filtered to remove undissolved solids. The filtrate was collected and the aqueous layer was washed with additional ether. The aqueous layer was acidified to pH 1-2, and the resulting solid was collected. The solid was then dissolved in ethyl acetate, dried, filtered and concentrated to provide the desired product (2.53 g, 81%) as a pale yellow solid.
[0567] Step B: Preparation of tert-butyl 6-cyano-7-fluoro-3,4-dihydro-2H-chromene-4-carboxylate: Making 6-cyano-7-fluoro-3,4-dihydro -2H-chromene-4-carboxylic acid was diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com