Chinese medicinal formula for treating heart disease
A technology of heart disease and formula, which is applied in the field of traditional Chinese medicine formula for the treatment of heart disease, can solve problems such as unsatisfactory treatment effect, temporary cure but not a permanent cure, narrow application range, etc., and achieve the effects of no toxic side effects, high cure rate, and short course of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
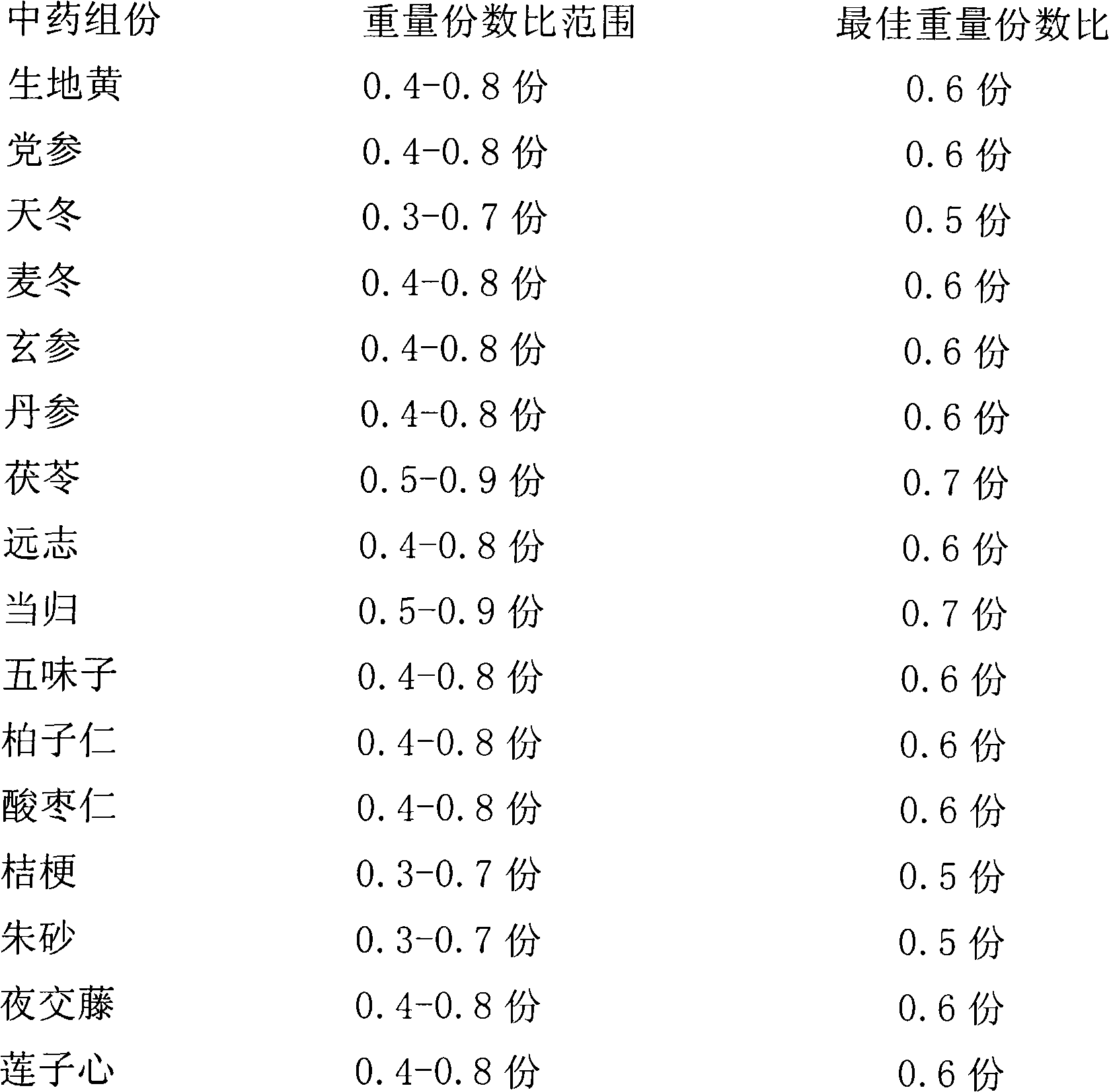
[0028] Take 0.4 parts of Rehmannia glutinosa, 0.4 parts of Codonopsis pilosula, 0.3 parts of Asparagus, 0.4 parts of Ophiopogon japonicus, 0.4 parts of Scrophulariaceae, 0.4 parts of Salvia miltiorrhiza, 0.5 parts of Poria cocos, 0.4 parts of polygala, 0.5 parts of angelica, 0.4 parts of Schisandra, 0.4 parts of Baiziren, 0.4 parts of wild jujube kernels, 0.3 parts of platycodon grandiflorum, 0.3 parts of cinnabar, 0.4 parts of Yejiao vine, and 0.4 parts of lotus seed heart.
[0029] After grasping according to the component content of the traditional Chinese medicine, decocting and taking it.
Embodiment 2
[0031] Take 0.6 parts of Rehmannia glutinosa, 0.6 parts of Codonopsis pilosula, 0.5 parts of Asparagus, 0.6 parts of Ophiopogon japonicus, 0.6 parts of Scrophulariaceae, 0.6 parts of Salvia miltiorrhiza, 0.7 parts of Poria cocos, 0.6 parts of polygala, 0.7 parts of angelica, 0.6 parts of Schisandra, 0.6 parts of Baiziren, 0.6 parts of jujube kernels, 0.5 parts of platycodon grandiflorum, 0.5 parts of cinnabar, 0.6 parts of Yejiao vine, and 0.6 parts of lotus seed heart.
[0032] After grasping according to the component content of the traditional Chinese medicine, decocting and taking it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com