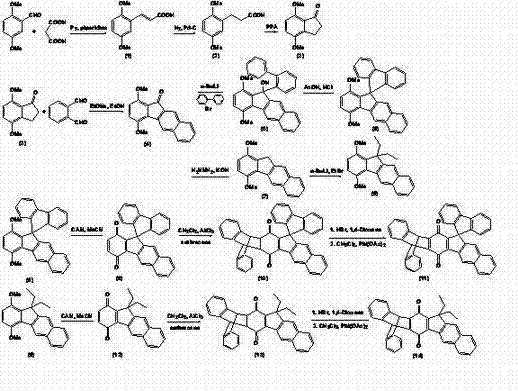
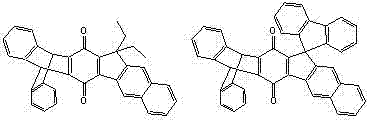
Benzoindenotriptycene and spirofluorene-benzoindenotriptycene derivatives and preparation method thereof
A technology of benzoindenotriptycene and triptycene, which is applied in the fields of benzoindenotriptycene and spirofluorene-benzoindenotriptycene derivatives and their preparation, which can solve development limitations and the difficulty of synthesis of dottycene quinone and other issues to achieve the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0024] Preparation of 2,5-dimethoxyphenylacrylic acid (1)
[0025] Mix 2,5-dimethoxybenzaldehyde (5.00 g, 30.00 mmol) and malonic acid (4.20 g, 40.00 mmol), add 12 ml of pyridine and 1 ml of piperidine, protect under nitrogen, and reflux at 110°C for 4 hours, the reaction solution was cooled and poured into a mixture of 100 ml of ice water and 5 ml of 2 N hydrochloric acid at 0°C to precipitate a white flocculent substance, which was filtered by suction to obtain a crude product, recrystallized with absolute ethanol, and dried to obtain a white Solid (5.48 g, 87.4%) (chloroform / methanol=20 / 1; R f = 0.43); M.p.: 144-145 oC; literature value: 151-152°C;
[0026] 1 H NMR (CDCl 3 , 500 MHz, ppm) δ:8.08 (d, J = 16.1 Hz, 1 H), 7.07 (s, 1 H), 6.94 (d, J = 8.9 Hz, 1 H), 6.87 (d, J = 9.0 Hz, 1 H), 6.52 (d, J = 16.1 Hz, 1 H), 3.86 (s, 3 H), 3.80 (s, 3 H).
[0027] Preparation of 2,5-dimethoxyphenylpropionic acid (2)
[0028]Dissolve 2,5-dimethoxyphenylacrylic acid (1) (20....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com