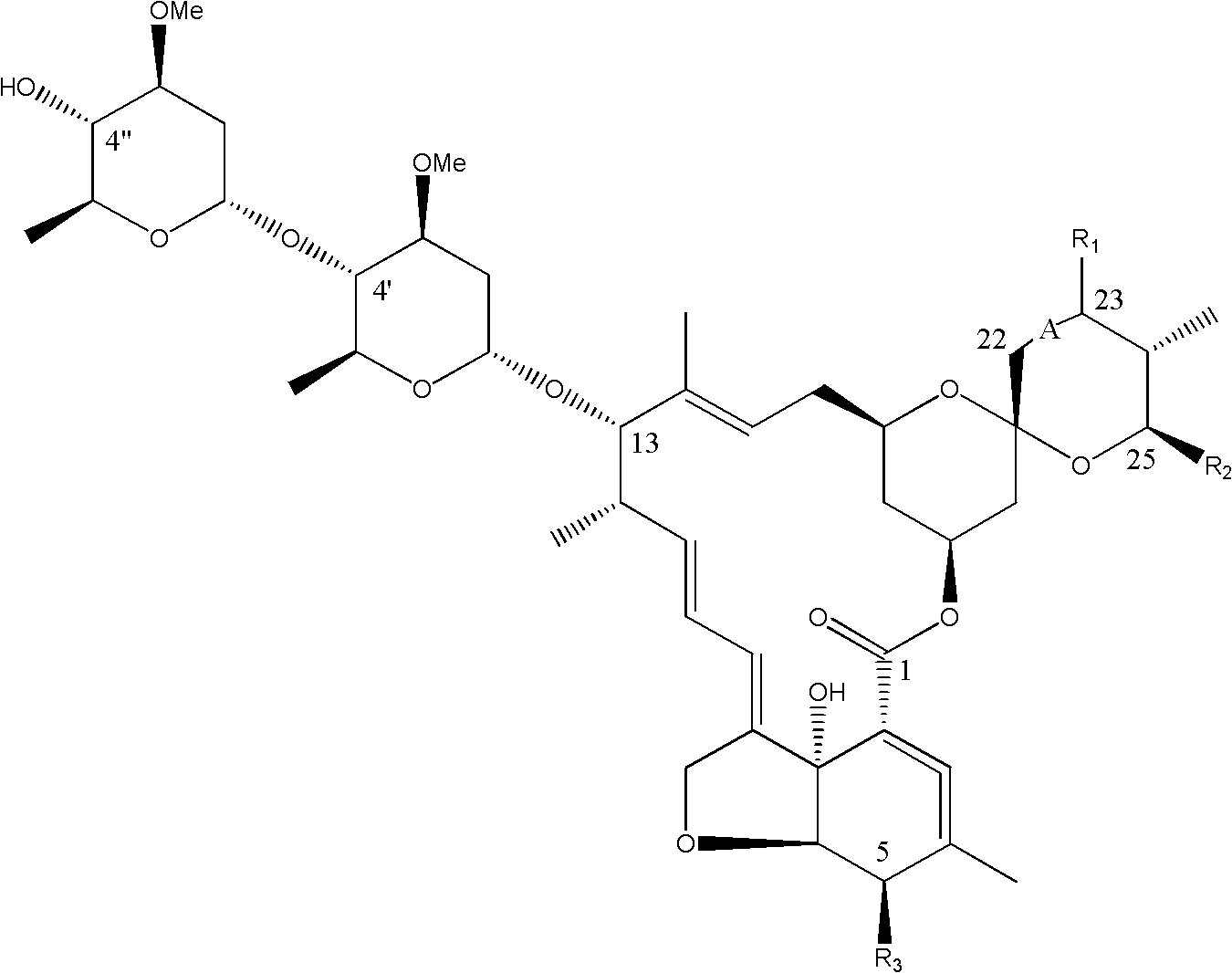
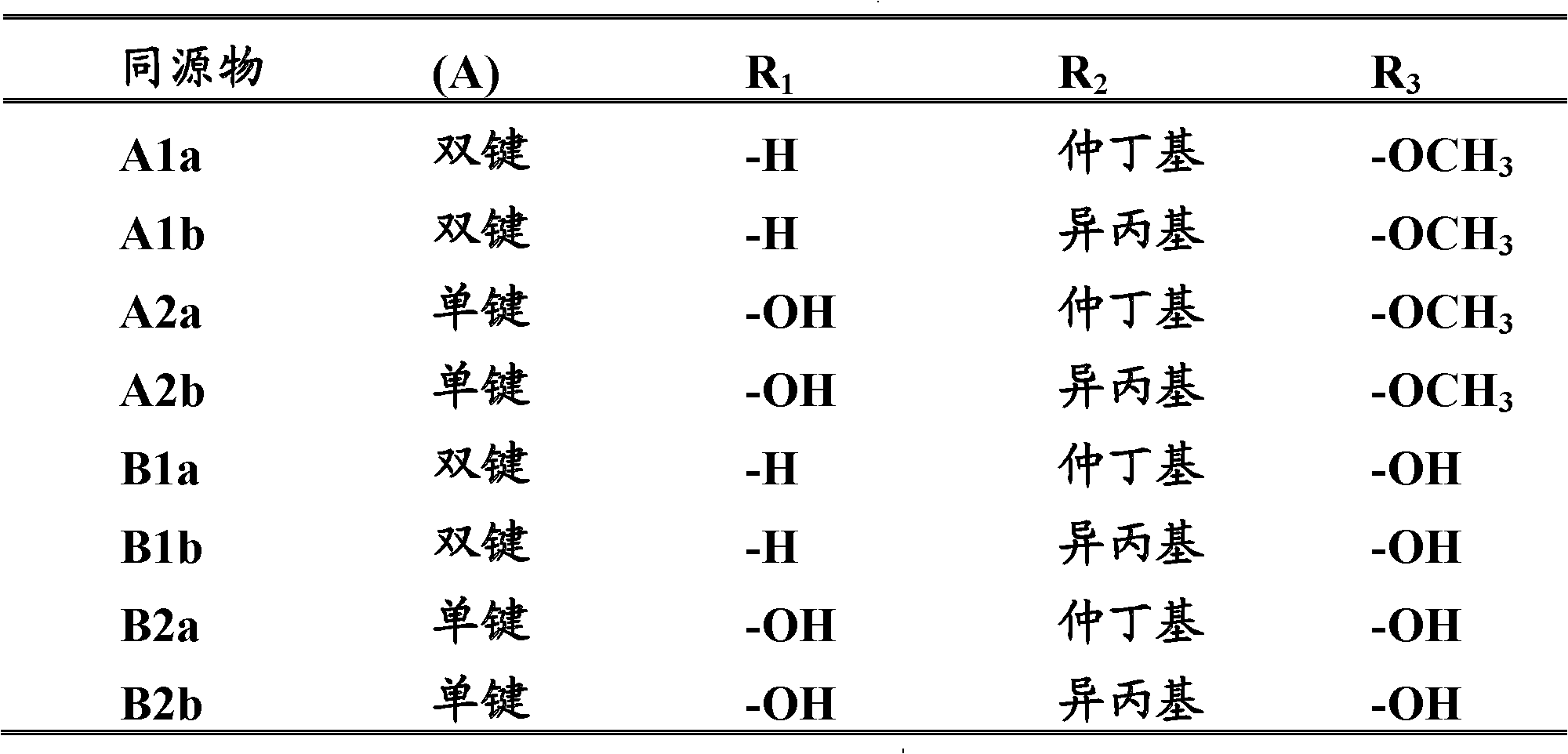
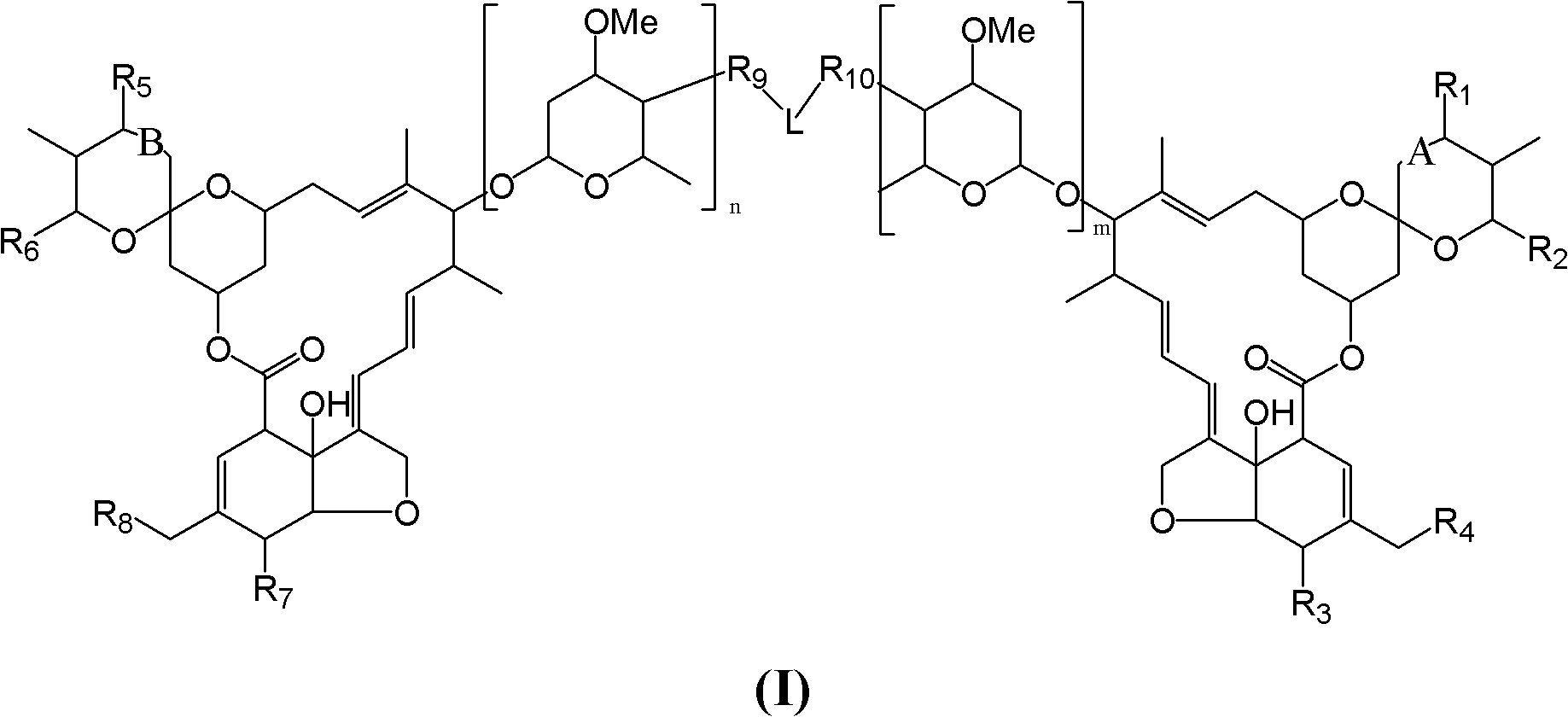
Dimeric avermectin and milbemycin derivatives
一种化合物、独立地的技术,应用在糖衍生物、药物组合、杂环化合物有效成分等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0699] In one embodiment, when the solution is applied to the back of the animal (eg along the backline, at one or more points), the direct drenching skin formulation according to the present invention can provide long-lasting and broad-spectrum efficacy. According to a first embodiment of the application of the direct-on-dose formulation, the method comprises applying the solution to the animal, repeating the application every month or every 2 months. According to a second embodiment of the application of the direct drench formulations, the method comprises applying the solution to livestock animals before they arrive at the feedlot, which application may be the last application before the animals are slaughtered. Obviously, the method can also be a combination of these two implementations, that is, the first one is followed by the second.
[0700] The solution according to the invention can be applied using any device known per se, for example using a dressing gun or a metering...
Embodiment 1
[0810]
[0811] The avermectin compound (XXXII) (which has a protected 5-hydroxyl group and a free 4"-hydroxyl group) can be reacted with a diiodide under alkaline conditions to form a dimeric compound. The Deprotection of 5-hydroxyl in the presence of reagents such as butylammonium can give the desired end product (XXXIII).
Embodiment 2
[0813]
[0814] Under reductive amination conditions, using dialdehyde compounds (XXXV), two molecules of 4"-epiamino avermectin derivatives (XXXIV) can be converted into dimerized products (XXXVI).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com