Modified protozoan expressing at least two variable surface proteins (vsp), its vaccine and its purification method, application and immunization method
A protozoa, surface protein technology, applied in biochemical equipment and methods, protozoa, protozoa antigen components, etc., can solve problems such as non-participation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
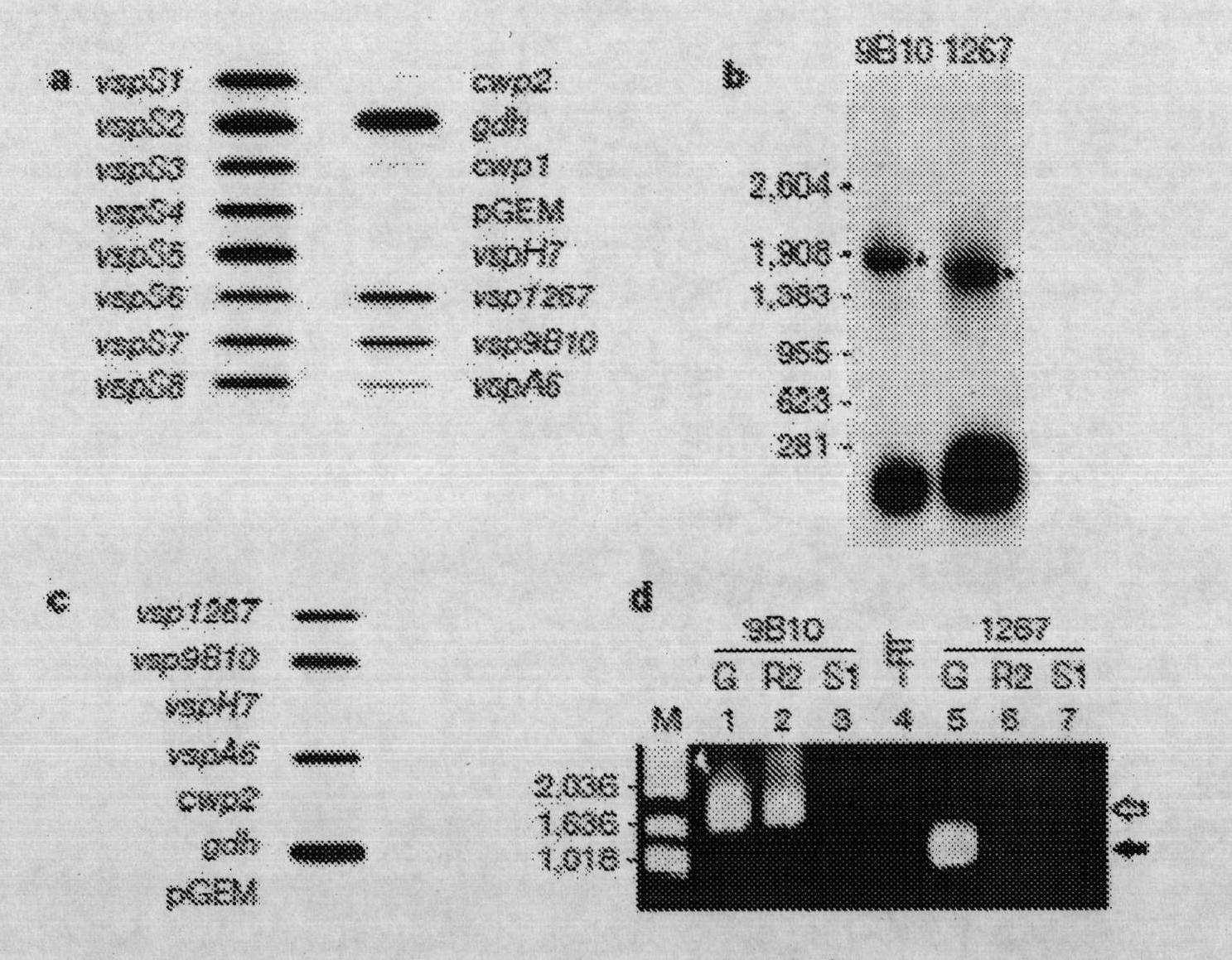
[0120] Example 1: Parasite culture and cloning: Giardia trophozoites (Lujan, H.D., Mowatt, M.R., Conrad, J.T., Bowers, B. and Nash, T.E.J. Biol. Chem. 270, 29307-29313 (1995). Serial cloning of trophozoites was performed by limiting dilution and selection based on immunofluorescence analysis using the corresponding anti-VSP monoclonal antibodies. Encapsulation was performed as previously reported ( Lujan, H.D., Mowatt, M.R., Conrad, J.T., Bowers, B., and Nash, T.E.I, J. Biol. Chem. 270, 29307-29313 (1995). WB clone 9B10 using Giardia lamblia trophozoites , 1267 and A6, and GS clone H7.
[0121] PCR: Isolation of Giardia Ramsey as described in (Mowatt, M.R.L., H.D.; Cotten, D.B.; Bowers, B.; Yee, J.; Nash, T.E.; Stibbs, H.H. Mol Microbiol 15, 955-63 (1995) Total DNA of flagellate trophozoites.
[0122] a: Sense primers S1 (5'-CVT GTG CHR RST GCA A-3') (SEQ ID No. 113), S2 (5'-TGC ACS RSC TGC YAB CC-3') (SEQ ID No. 114), S3 (5'-TAG TGY DSY VMV TGY AA-3') (SEQ ID No. 115) and ...
Embodiment 2
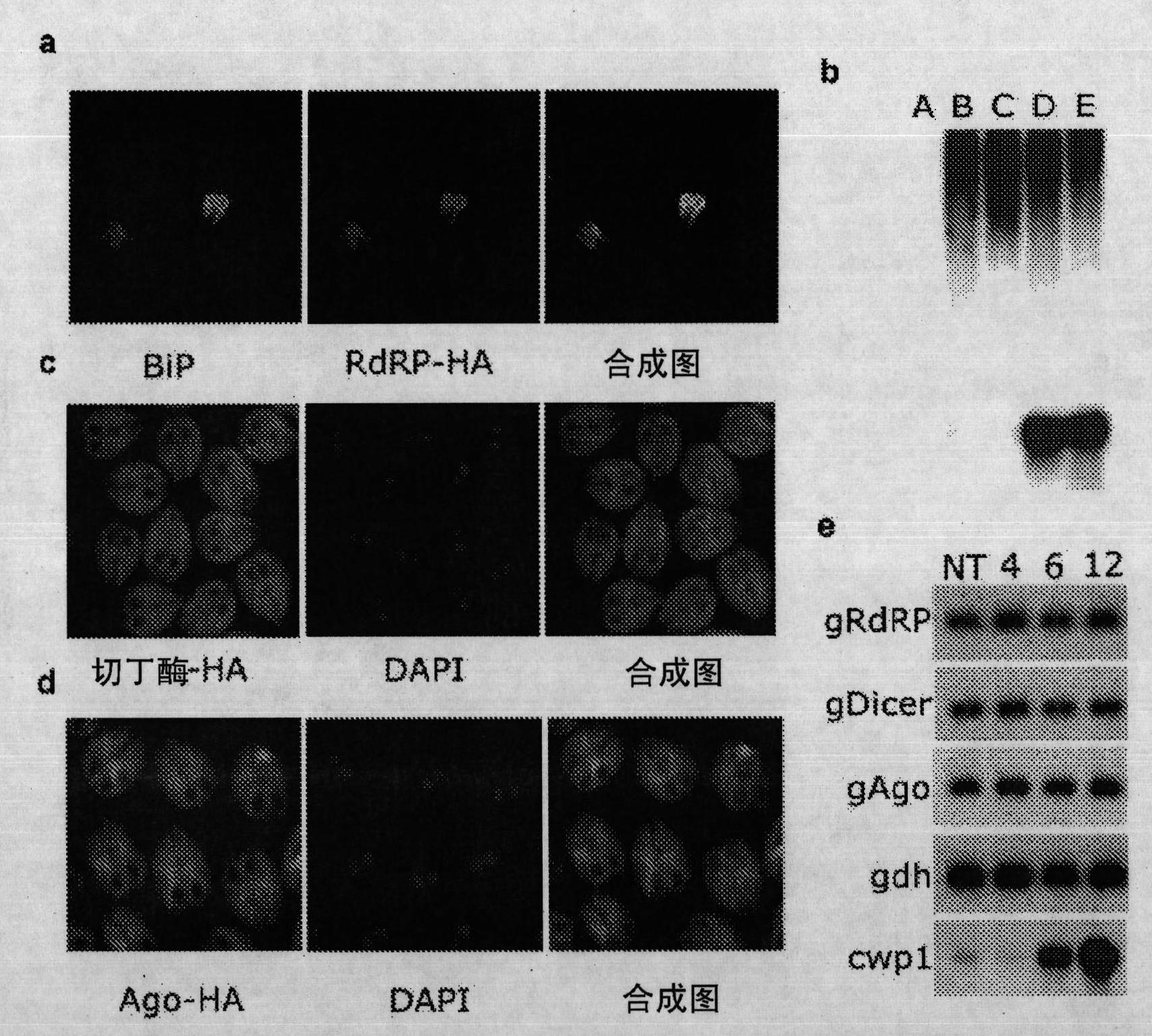
[0132] Generation of monoclonal antibodies against VSP 5 amino acids as well as against individual VSPs: 200 mg of (a) NH conjugated to KLH via sMBS cross-linker was used 2 - HPLC-purified preparation of CRGKA-COOH peptide, or (b) synthetic polyantigen peptide [NH 2 -CRGKA] 8 -[K] 7 -bAla-OH (both from Biosynthesis, Inc.), or protein extracts of cultured trophozoites derived from WB isolates emulsified in the Sigma adjuvant system (Sigma), subcutaneously immunized 6-week-old females BALB / c mice. After 21 days, 200 mg of the same formulation was boosted subcutaneously, and 20 days later, 100 mg of the antigen formulation was boosted intravenously. After 3 days, mice were sacrificed and splenocytes were used for fusion with NSO myeloma cells. Screening of antibody-secreting hybridomas by ELISA using native peptides, or by indirect immunofluorescence using unencapsulated and encapsulated trophozoites (Jambhekar, A.D. et al., RNA 13, 625-642 (2007) and Aggarwal, A. , Merritt,...
Embodiment 3
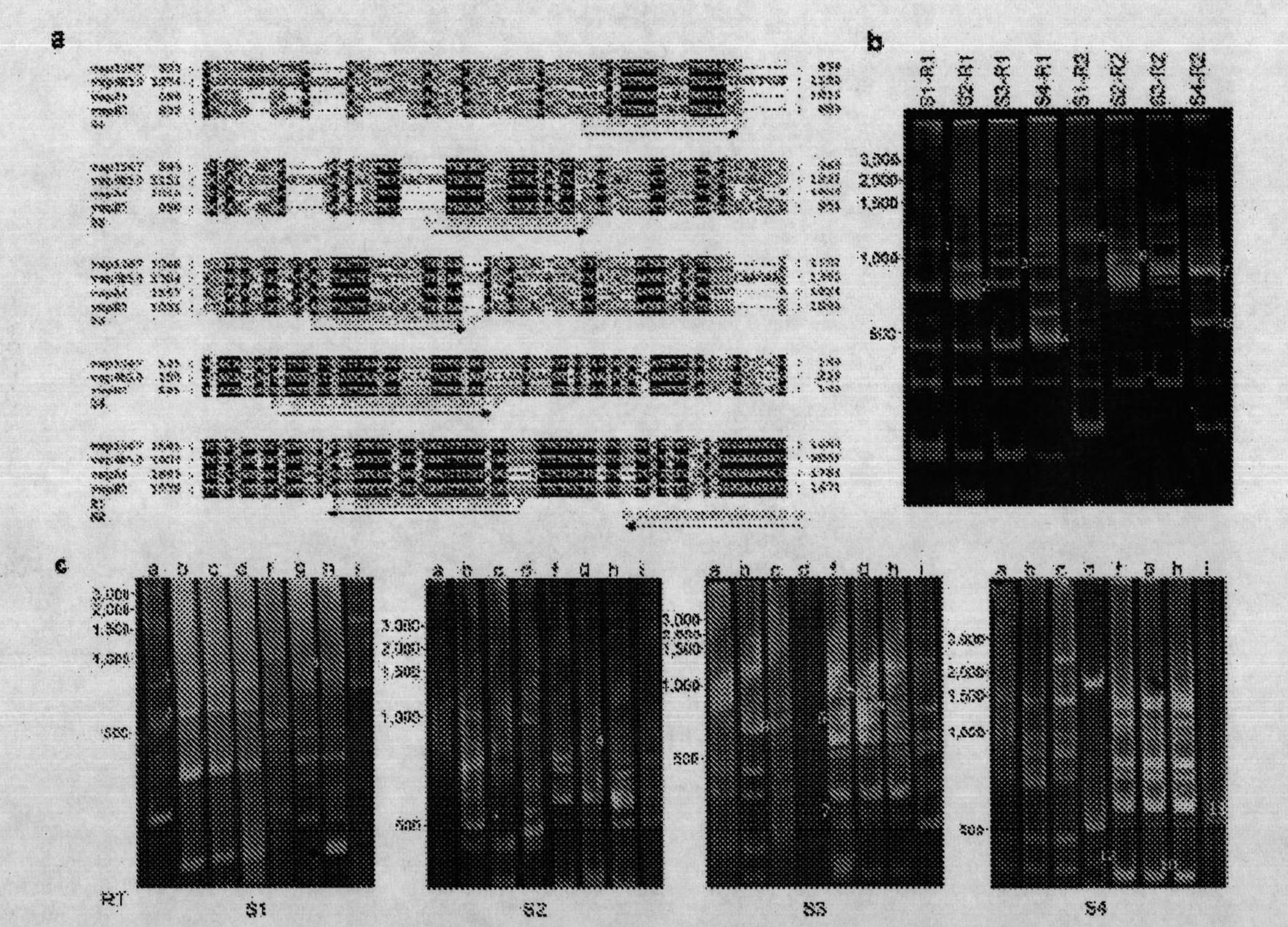
[0134] Example 3: Preparation and Protection Analysis of Different Vaccines
[0135] 1. Parasites: Antigenic analysis of Giardia lamblia from Afghanistan, Puerto Rico, Ecuador, and Oregon from symptomatic patients diagnosed with giardiasis WC strain (ATCC 30957) . Smith PD, Gillin FD Kaushal NA and Nash TE. Infect. Immun. 1982 May; 36(2): 714-9), isolation of GS / M strains from symptomatic patients in the United States (Antigenic analysis of Giardia lamblia from Afghanistan, Puerto Rico, Ecuador, and Oregon. Smith PD, Gillin FD Kaushal NA and Nash TE. Infect. Immun. 1982 May;36(2):714-9); clones and transgenic trophozoites derived from WB strains were grown at 37°C in TYI-S-33 medium supplemented with 20% bovine serum (Invitrogen), bovine bile (Sigma) and antibiotic / antifungal solution in 12ml borosilicate glass bottles with screw caps (Methods for cultivation of luminal parasitc protists of clinical importance. Clark CG, Diamond LS. Clin Microbiol Rev. 2002 Jul;15(3):329-41)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com