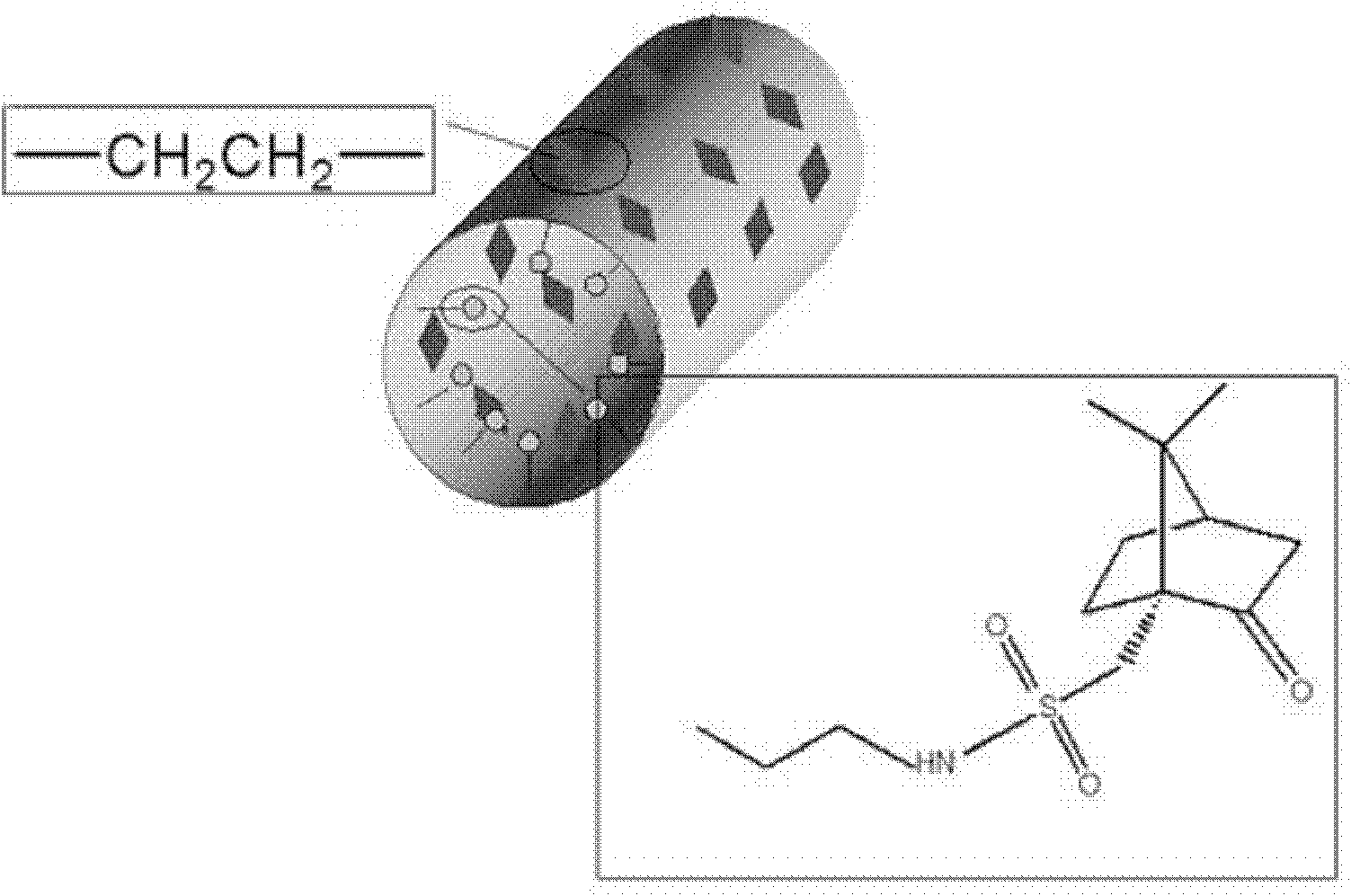
A camphorsulfonamide-doped ethane bridge spherical mesoporous silica gel
A mesoporous silica gel, sulfonamide technology, applied in other chemical processes, chemical instruments and methods, etc., can solve the problems of harsh conditions, difficult to control the uniformity of organic group distribution, and complex processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of Camphorsulfonamide-doped Spherical Mesoporous Silica Gel with Ethane Bridges
[0030] Take by weighing 0.94g octadecyltrimethylammonium chloride in a 100ml round bottom flask, add deionized water (21.0ml), ethanol (18.0ml) and sodium hydroxide solution (1M, 13.5ml) successively, stir vigorously to make Mix well to obtain a clear and transparent solution. The ethanol solution of 1,2-bis(triethoxysilyl)ethane (1.59g) and camphorsulfonamide derivative silicon ester I (0.39g) mixed uniformly was added dropwise to the above-mentioned transparent solution, at room temperature Stirring was continued for 1 hour, and then the reaction solution was transferred to a reaction kettle and stood at 90° C. for 18 hours, filtered, thoroughly washed with deionized water, and dried to obtain a solid material. Mix 1 g of solid material with 200 ml of acidic ethanol and extract at 60°C for 6 hours to remove the template agent, filter, wash and dry to obtain camphorsulfonamide-...
Embodiment 2
[0032] Application of Camphorsulfonamide-doped Spherical Mesoporous Silica Gel as Chromatographic Stationary Phase
[0033] Get the camphorsulfonamide-doped ethane bridge bonded spherical mesoporous silica gel prepared in Example 1, use the homogenization method to fill in a stainless steel column tube (150mm × 4.6mm, I.D.), the column packing pressure is 40MPa, and use this chromatographic The column was tested for chromatographic separation. A Shimadzu LC-20AT high performance liquid chromatograph and an SPD-20A ultraviolet detector were selected, and the probe molecules were selected from benzene, anthracene and nitrobenzene, all of which were prepared into a solution of about 200 μg / ml with n-hexane as a solvent, and the injection volume was 20 μL. The mobile phase is n-hexane, the flow rate is 1ml / min, and the detection wavelength is 254nm. The three compounds can achieve baseline separation. Chromatogram see Figure 8 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap