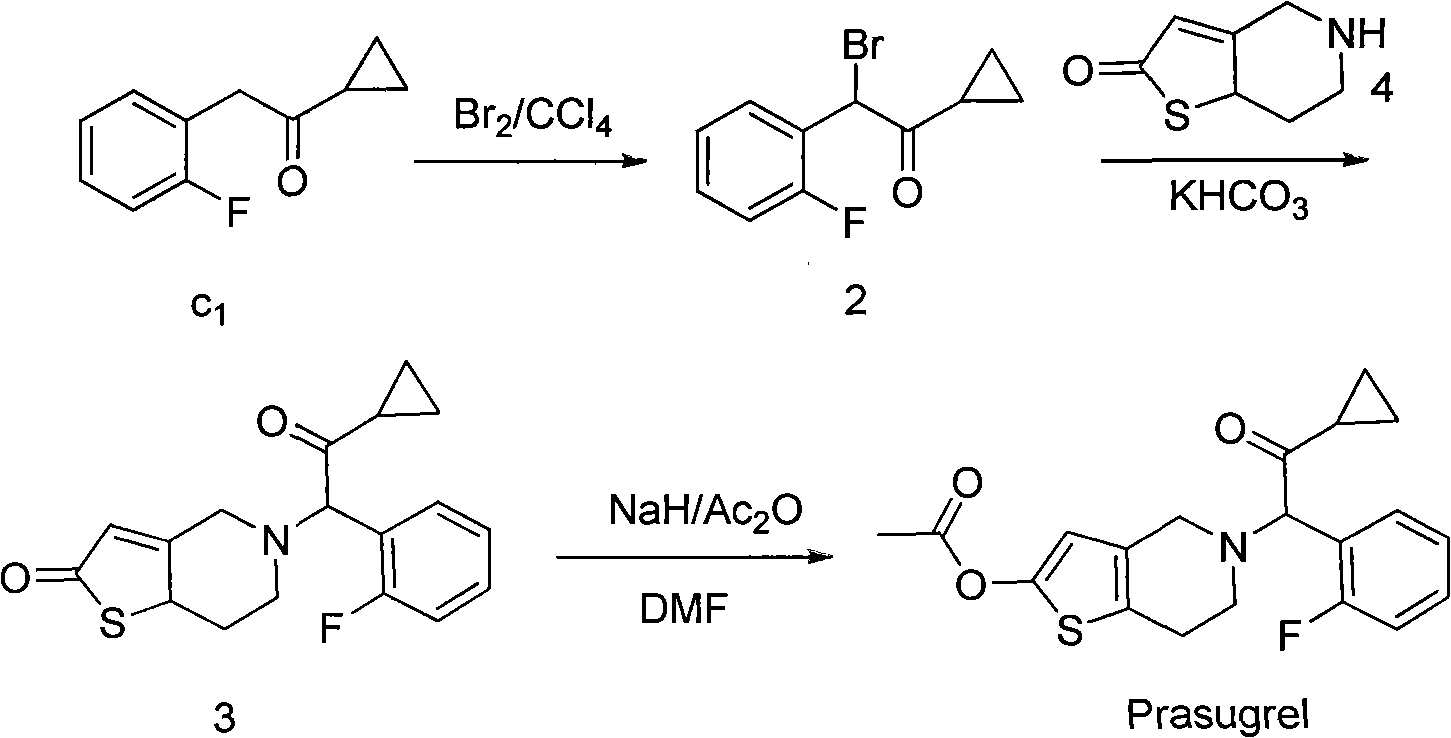
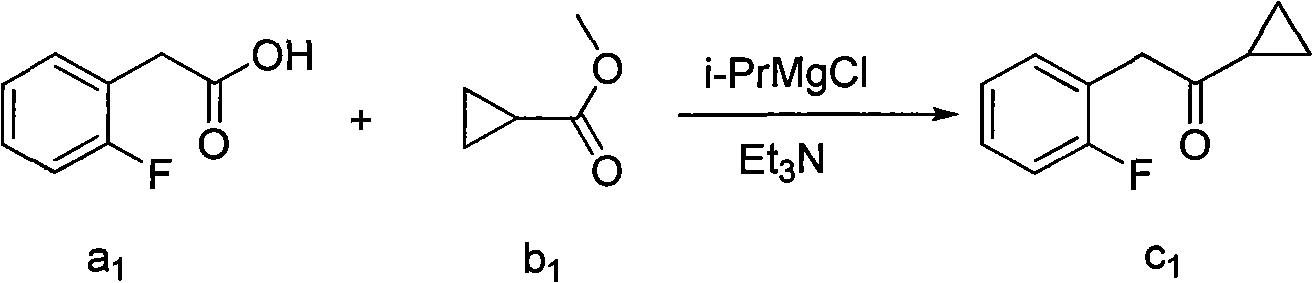
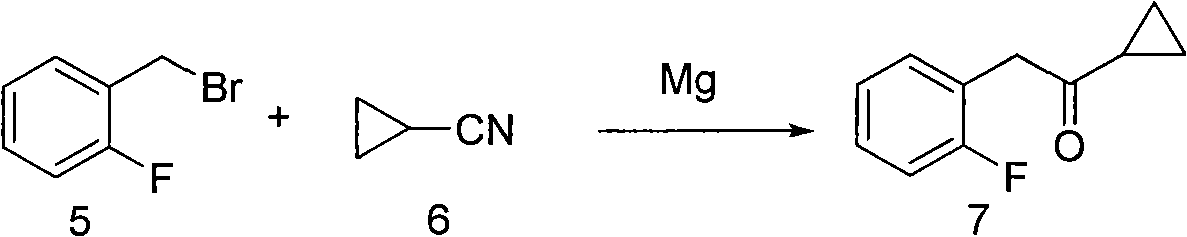Preparation method of aromatic cyclopropyl ketone compound and purpose
A technology for cyclopropyl ethyl ketone and compounds, which is applied in the direction of condensation preparation of carbonyl compounds, organic chemistry, etc., can solve the problems of difficult post-processing, toxicity of reaction solvents, serious environmental pollution, etc., and achieves high industrial application, economic value, and low price. , the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of 1-cyclopropyl-2-(2-fluorophenyl)ethanone
[0025] Add 2-(2-fluorophenyl)acetic acid (10.0g, 64.9mmol) into 30ml of anhydrous tetrahydrofuran, add dropwise to the cloudy solution containing sodium hydride (3.9g, 97.4mmol) and 140mL of tetrahydrofuran at 10°C, drop After completion, the reaction mixture was heated to 30°C and stirred for 1 hour, then a mixed solution of methylcyclopropylcarboxylic acid (7.0ml, 69.8mmol, 1.07eq) and 30ml of anhydrous tetrahydrofuran was added dropwise, and stirring was continued for 10 hours after the dropwise addition was completed. After the reaction is completed, add ammonium chloride solution dropwise to quench excess sodium hydride, separate liquids, extract, concentrate the organic phase, and distill the resulting liquid under reduced pressure to obtain 1-cyclopropyl-2-(2-fluorophenyl)ethanone 10.8 g, yield 93.5%.
[0026] 1 H NMR (CDCl 3 , Me 4 Si) δ0.86-0.90 (m, 2H), 1.05-1.08 (m, 2H), 1.97-2.02 (m, 1H...
Embodiment 2
[0027] Example 2: Preparation of 1-cyclopropyl-2-(2-fluorophenyl)ethanone
[0028] Add 2-(2-fluorophenyl)acetic acid (10.0g, 64.9mmol) into 30ml of anhydrous methanol, dropwise add sodium methoxide (4.6g, 85mmol) and 100ml of methanol into the turbid solution at 10°C, dropwise The reaction mixture was heated to 30°C and stirred for 1 hour, then a mixture of methylcyclopropylcarboxylic acid (7.0ml, 69.8mmol, 1.07eq) and 30ml of methanol was added dropwise, and stirring was continued for 10 hours after the completion of the dropwise addition. Add ammonium chloride solution to quench excess sodium methoxide, separate liquids, extract, and concentrate the organic phase. The resulting liquid is distilled under reduced pressure to obtain 9.4 g of 1-cyclopropyl-2-(2-fluorophenyl)ethanone. The rate is 81.3%.
Embodiment 3
[0029] Embodiment 3: Preparation of 1-cyclopropyl-2-(3-tolyl)ethanone
[0030] Take by weighing 2-(3-toluene) acetic acid 10.0g, sodium hydride 4.0g, operate in the same way according to the method of embodiment 1, obtain 1-cyclopropyl-2-(3-toluene) ethyl ketone 9.18g, yield is 79.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com