Manufacturing method of developable inflatable balloon for kyphoplasty
A technology of kyphoplasty and kyphoplasty, which is applied in the field of medical devices, can solve the problems that affect the doctor's accurate judgment of inflatable balloons, limit the application of polyurethane imaging materials, and poor development of inflatable balloons, etc., and achieve excellent imaging effects , good cell compatibility, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The pre-dehydration-treated poly(1,6-hexanediol) diol with a molecular weight of 1500 and 4'4-diphenylmethane diisocyanate are mixed in a molar ratio of 0.39:1, N 2 Stir and react at 80°C for 2.5 hours under protection to obtain a prepolymer, heat up to 90°C, add molten chain extender N,N-dihydroxyethyl-p-iodoaniline, the molar ratio of chain extender to diisocyanate is 0.3:1, Stir for 20 minutes, and plasticize at 75°C for 5 hours after discharge to obtain developable polyurethane with a hardness of 90A.
[0027] After vacuum-drying the developable polyurethane with a hardness of 90A at 50°C for 4-5 hours, add it to the extruder and extrude it into a transparent, smooth surface, and bubble-free wall thickness with an inner diameter of 2mm and an outer diameter of 3mm Uniform balloon tubing. The extrusion conditions are: the temperature of the feeding section of the extruder barrel is 175°C, the temperature of the compression section is 180°C, the temperature of the me...
Embodiment 2
[0030] The pre-dehydration-treated polytetrahydrofuran ether diol with a molecular weight of 2000 and 4'4-diphenylmethane diisocyanate are mixed in a molar ratio of 0.5:1, N 2 Stir and react at 80°C for 2 hours under protection to obtain a prepolymer, heat up to 85°C, add molten chain extender N,N-dihydroxyethyl p-iodoaniline, the molar ratio of chain extender to diisocyanate is 0.2:1, Stir for 10 minutes, and plasticize at 75°C for 5 hours after discharging to obtain developable polyurethane with a hardness of 80A.
[0031] The developable polyurethane with a hardness of 80A is vacuum-dried at 50°C for 4-5 hours, then added to the extruder, and extruded into a transparent, smooth surface and no bubbles with an inner diameter of 2.5mm and an outer diameter of 3.5mm Tubing for balloons with uniform wall thickness. The extrusion conditions are: the temperature of the feeding section of the extruder barrel is 185°C, the temperature of the compression section is 190°C, the temper...
Embodiment 3
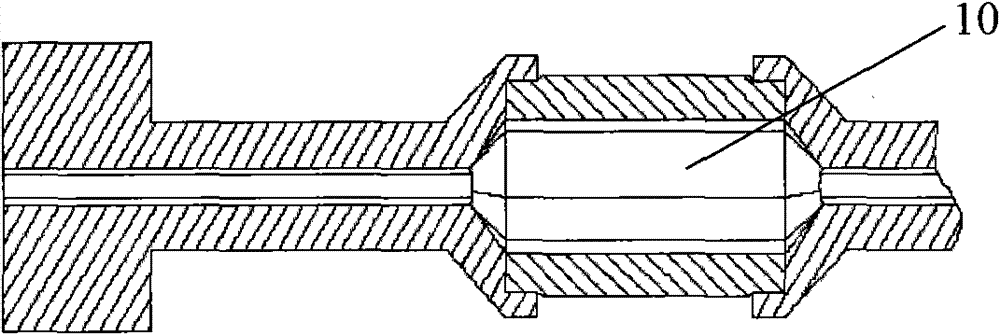
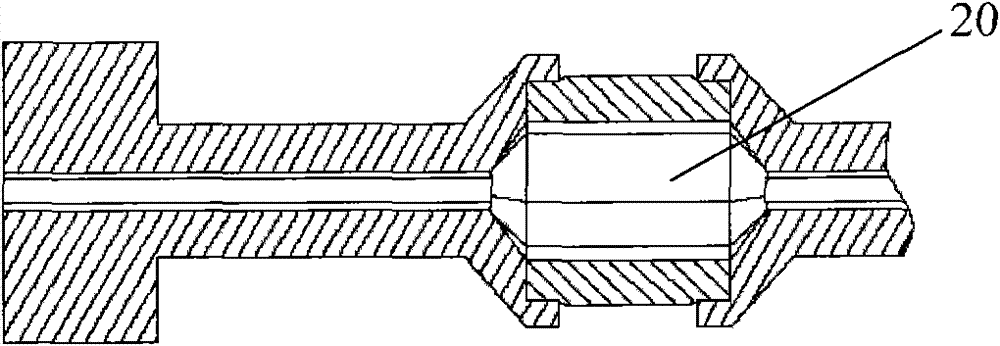
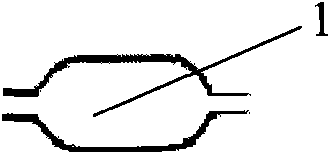
[0034] Such as Figure 4 As shown, the RQ13 vertebral balloon catheter is composed of a Y-shaped head 3, a main catheter 2, a balloon 1, and a core tube 4. The specific assembly is as follows: the lower end of the balloon 1 is sealed with the lower end of the core tube 4 with an outer diameter of 1.9mm, The upper end of the balloon 1 with an inner diameter of 2.2 mm is sealed with the lower end of the main catheter 2 with an outer diameter of 2.1 mm. The upper end of the main catheter 2 is inserted into the sheath tube and connected to the Y-shaped head 3. The guide wire is placed in the Y-shaped head 3, and the assembly is completed.
[0035]During the operation, the patient lies prone, and the body surface projection of the pedicle of the diseased vertebra is monitored and determined by the C-arm X-ray machine. Expand enough for the inflatable balloon to pass through to establish a working channel; insert the visualized inflatable balloon of the RQ13 vertebral body catheter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com