Ni-Cu binary catalyst for improving performance of direct borohydride fuel cell
A fuel cell and catalyst technology, applied in the field of electrochemical applications, can solve the problems of low direct oxidation performance, large charge transfer resistance, low fuel utilization rate, etc., so as to enhance the direct oxidation performance, reduce the charge transfer resistance, and improve the discharge. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Under normal pressure, the temperature is in the range of 293.15 ~ 313.15K, and 0.25mol / L CuSO is configured under acidic conditions 4 solution. 2cm 2 The Ni sheet was used as the working electrode and placed in the above solution, the Cu rod was used as the counter electrode, and the silver / silver chloride electrode was used as the reference electrode. On the electrochemical workstation, the constant potential method was used to electrodeposit at -0.1V for 5s. A Ni-Cu binary catalyst was obtained. Weigh sodium borohydride, and dissolve it in 2mol / L sodium hydroxide solution to prepare 0.27mol / L sodium borohydride solution, mix well and use it as electrolyte for direct borohydrogen fuel cell. 2cm 2 The Ni sheet and Ni-Cu were used as the working electrode, the mercury / mercury oxide electrode was used as the reference electrode, and the graphite rod was used as the auxiliary electrode. Cyclic voltammetry was used for performance testing.
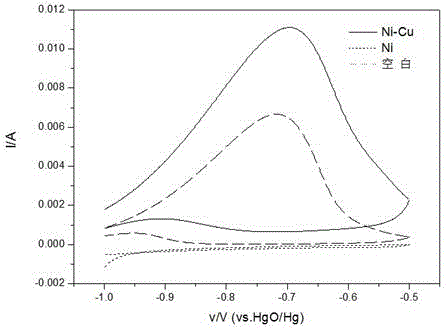
[0016] figure 1 gives NaBH...
Embodiment 2
[0019] Under normal pressure, the temperature is in the range of 293.15 ~ 313.15K, and 0.25mol / L CuSO is configured under acidic conditions 4 solution. 2cm 2 The Ni sheet was used as the working electrode and placed in the above solution, the Cu rod was used as the counter electrode, and the silver / silver chloride electrode was used as the reference electrode. On the electrochemical workstation, the constant potential method was used to electrodeposit at -0.1V for 5s. A Ni-Cu binary catalyst was obtained. Weigh sodium borohydride, and dissolve it in 2mol / L sodium hydroxide solution to prepare 0.27mol / L sodium borohydride solution, mix well and use it as electrolyte for direct borohydrogen fuel cell. 2cm 2 The Ni sheet and Ni-Cu are used as the working electrode, the mercury / mercury oxide electrode is used as the reference electrode, and the graphite rod is used as the auxiliary electrode, and the AC impedance spectroscopy performance test is performed.
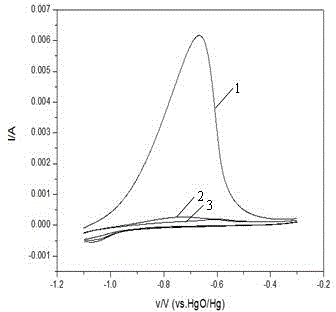
[0020] In the AC i...
Embodiment 3
[0022] Under normal pressure, the temperature is in the range of 293.15 ~ 313.15K, and 0.25mol / L CuSO is configured under acidic conditions 4 solution. 2cm 2 The Ni sheet was used as the working electrode and placed in the above solution, the Cu rod was used as the counter electrode, and the silver / silver chloride electrode was used as the reference electrode. On the electrochemical workstation, the constant potential method was used to electrodeposit at -0.1V for 5s. A Ni-Cu binary catalyst was obtained. Weigh sodium borohydride, and dissolve it in 2mol / L sodium hydroxide solution to prepare 0.27mol / L sodium borohydride solution, mix well and use it as electrolyte for direct borohydrogen fuel cell. 2cm 2 The Ni sheet and Ni-Cu are used as the working electrode, the mercury / mercury oxide electrode is used as the reference electrode, and the graphite rod is used as the auxiliary electrode, and the constant current discharge performance test is carried out.
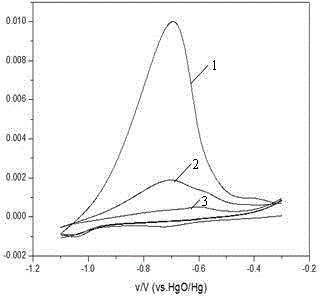
[0023] Fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com