Preparation method of carbazole schiff base copolymer
A Schiff base and copolymer technology, applied in the field of polymer electroluminescent materials, can solve the problems of fluorescence quenching, luminous efficiency and brightness reduction, and achieve the effects of low generation temperature, no pollution to the environment, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Weigh 5g carbazole and 15g KOH, dissolve carbazole in 100ml of N'N-dimethylformamide, add KOH, heat the system to 50°C under mechanical stirring, and use a constant pressure dropper Add 5.8 g of bromooctane, react at room temperature for 20 h, extract the resulting reaction solution with dichloromethane, wash the extract with distilled water until pH = 7, dry the resulting extract with anhydrous magnesium sulfate, then filter, and finally Remove the dichloromethane solvent by rotary evaporation to obtain orange-yellow N-n-octylcarbazole;
[0016] (2) Weigh 5.0g N-octylcarbazole, then dissolve it in 20ml redistilled N'N-dimethylformamide, stir and mix well, and slowly add 315ml dropwise with a constant pressure dropper under ice-water bath conditions After reacting for 1 hour, heat up to 100°C and stir for 3 hours. After the reaction solution is cooled, neutralize it with NaOH solution to pH = 7, then extract it with dichloromethane, wash it with water three times, a...
Embodiment 2
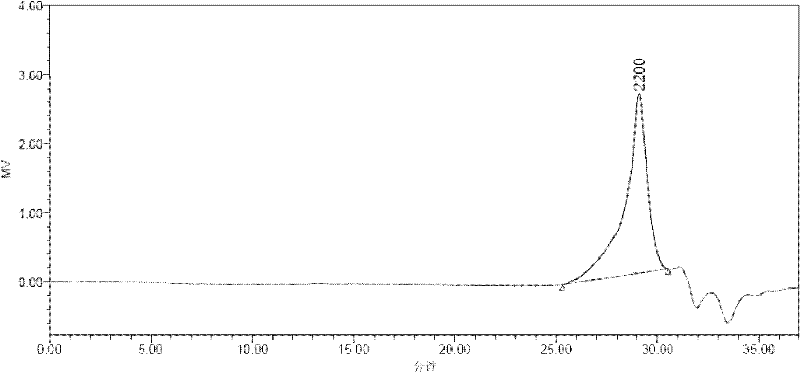
[0020] The operating method is the same as in Example 1, except that the third step reaction time is extended from 12 hours to 24 hours. The analysis results show that the number average molecular weight in the product is 7645, the weight average molecular weight is 9628, and the polydispersity is 1.25. Other properties, such as DSC and PL tests, were unchanged.
Embodiment 3
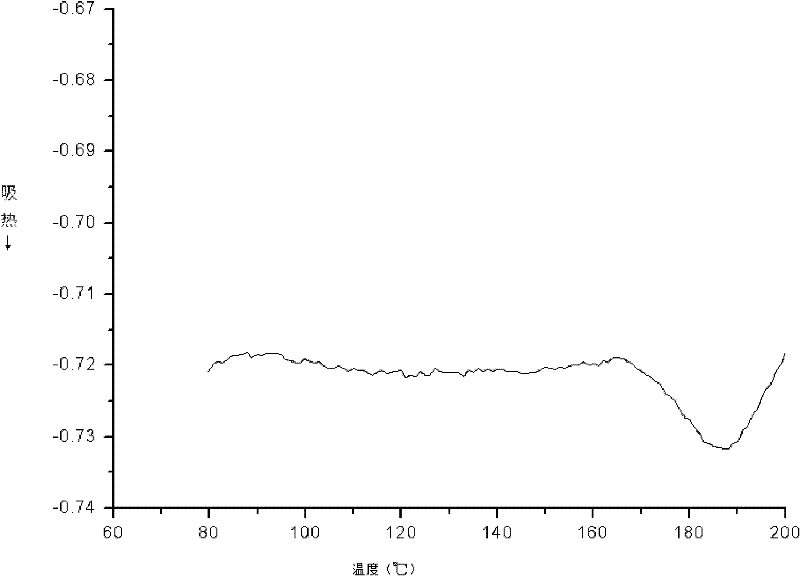
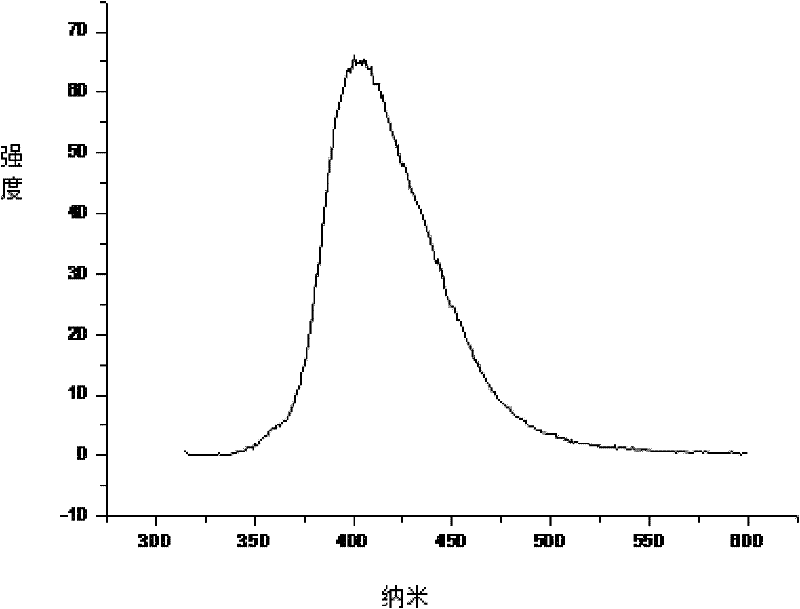
[0022] The operation method is the same as in Example 1, except that in the first step, the reaction temperature is increased from 50°C to 70°C, and the reaction time is extended from 12 hours to 24 hours during the third step, and the analysis results show that the number average molecular weight in the product is 8650 , the weight average molecular weight is 11071, and the polydispersity is 1.27. Other properties, such as DSC and PL tests, were unchanged. In the PL test, the maximum fluorescence peak is at 401nm. No change in DSC testing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com