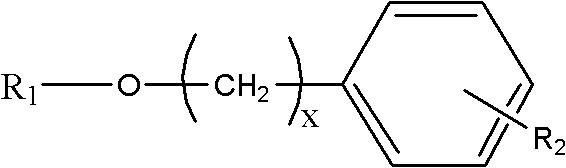Salidroside analogues as well as preparation method and application thereof
A technology of salidroside and analogs, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of poor regeneration ability, complex extraction process, reduction of wild resource reserves, etc., and achieve cost reduction. , the effect of shortening the reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
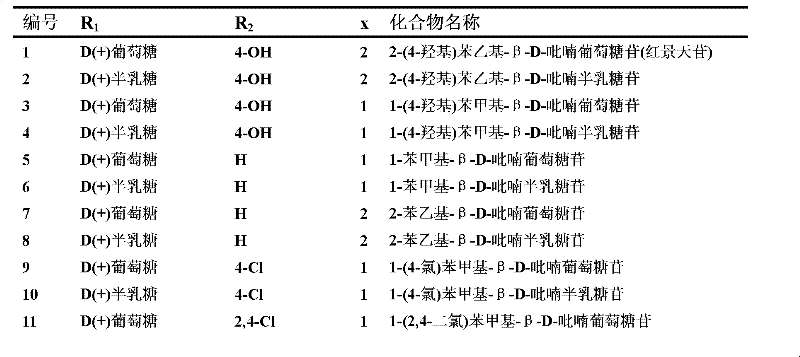
[0043] Example 1: Preparation of 1-(4-hydroxy)phenethyl-β-D-glucopyranoside (salidroside, Compound No. 1).
[0044] In a 50ml round bottom flask, add 15ml (2:1) of a mixed solvent of anhydrous dichloromethane and ether, 0.55g (4.0mmol) of tyrosol, 1.2g (4.5mmol) of silver carbonate, and a small amount of iodine (0.1-0.5ml) , 2g of powdered molecular sieves, stirred in the dark for 30min under the protection of nitrogen, added 1.95g (5mmol) of 2,3,4,6-tetra-O-acetyl-α-D-bromoglucopyranose, and reacted at room temperature for 10h. Filter, evaporate the solvent under reduced pressure to obtain a viscous colorless syrup, add 15ml of methanol solution dissolved in potassium carbonate, react at room temperature for 6h, filter, neutralize the acidic resin, separate by column chromatography, methanol:dichloromethane=1: 5. Obtained 0.98g of white solid compound 1, mp159-160°C, yield 81.6%.
Embodiment 2
[0045] Example 2: Preparation of 1-(4-chloro)benzyl-β-D-galactopyranoside (compound 10).
[0046] In a 50ml round-bottomed flask, add 15ml (2:1) of a mixed solvent of anhydrous dichloromethane and ether, 0.57g (4mmol) of 4-chlorobenzyl alcohol, 1.2g (4.5mmol) of silver carbonate, and a small amount of iodine (0.1-0.5 ml), powdered molecular sieve 2g, under the protection of nitrogen, stir in the dark for 30min, add 1.95g (5mmol) of 2,3,4,6-tetra-O-acetyl-α-D-bromoglucopyranose, and react at room temperature for 10h . Filter, evaporate the solvent under reduced pressure to obtain a viscous colorless syrup, add 15ml of methanol solution dissolved in potassium carbonate, react at room temperature for 6h, filter, neutralize the acidic resin, separate by column chromatography, methanol:dichloromethane=1: 6. Obtained 0.95 g of light yellow solid of compound 10, mp 138-140°C, yield 78.1%.
Embodiment 3
[0047] Example 3: Preparation of 1-(3,5-dimethoxy)benzyl-β-D-glucopyranoside (Compound 19).
[0048] In a 50ml round bottom flask, add 15ml (2:1) of a mixed solvent of anhydrous dichloromethane and ether, 0.67g (4mmol) of 3,5-dimethoxybenzyl alcohol, 1.2g (4.5mmol) of silver carbonate, iodine A small amount (0.1~0.5ml), 2g of powdered molecular sieve, stirred for 30min in the dark under the protection of nitrogen, added 1.95g (5mmol ), react at room temperature for 10h. Filter, evaporate the solvent under reduced pressure to obtain a viscous colorless syrup, add 15ml of methanol solution dissolved in potassium carbonate, react at room temperature for 6h, filter, neutralize the acidic resin, separate by column chromatography, methanol:dichloromethane=1: 4. Obtained 1.08 g of white solid compound 19, 146-148 °C, yield 81.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com