Liquid dressing containing chitosan derivative and preparation method thereof
A technology of chitosan derivatives and liquid dressings, which is applied in the field of medical devices to achieve the effects of easy use, high transparency, and promotion of wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Get each component by following weight percentage:
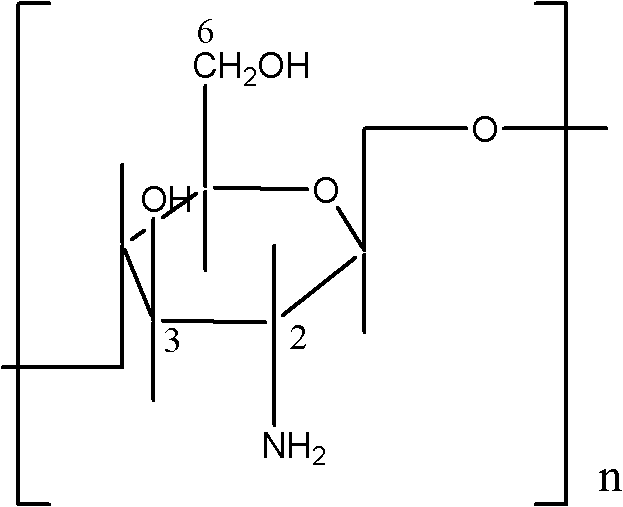
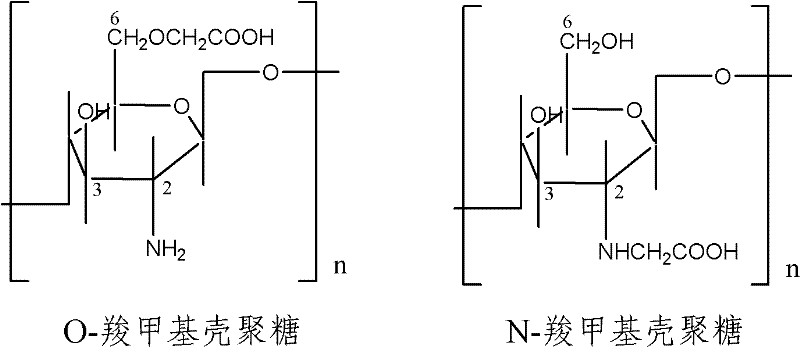
[0044] Based on 1000g of liquid dressing, carboxymethyl chitosan 1%, collagen powder 0.5%, glycerin 10%, triclosan 0.2%, azone 5%, and the rest is deionized water.
[0045] Preparation:
[0046] 1) Weigh 10 g of carboxymethyl chitosan, add it into 350 g of deionized water and stir until completely dissolved to form liquid A, and leave it in place;
[0047] 2) Weigh 5g of collagen powder, add it into 200g of deionized water and stir until completely dissolved to form liquid B, keep it;
[0048] 3) Weigh 50 g of azone and 2 g of triclosan in sequence, dissolve them in 100 g of glycerol successively, and mix them uniformly to form liquid C;
[0049] 4) Pour liquids B and C into liquid A in turn and mix evenly to form mixed liquid D, then add the remaining deionized water and stir well until they are evenly mixed;
[0050] 5) Filling.
Embodiment 2
[0052] Get each component by following weight percentage:
[0053] Based on 1000g of liquid dressing, carboxymethyl chitosan 2%, collagen powder 0.4%, glycerol 12%, triclosan 0.25%, azone 8%, and the rest is deionized water.
[0054] Preparation:
[0055] 1) Weigh 20g of carboxymethyl chitosan, add it into 350g of deionized water and stir until completely dissolved to form liquid A, and leave it in place;
[0056] 2) Weigh 4g of collagen powder, add it to 200g of deionized water and stir until completely dissolved to form liquid B, keep it;
[0057] 3) Weigh 80 g of azone and 2.5 g of triclosan in sequence, dissolve them in 120 g of glycerol successively, and mix them uniformly to form liquid C;
[0058] 4) Pour liquids B and C into liquid A in turn and mix evenly to form mixed liquid D, then add the remaining deionized water and stir well until they are evenly mixed;
[0059] 5) Filling.
Embodiment 3
[0061] Get each component by following weight percentage:
[0062] Based on 1000g of liquid dressing, carboxymethyl chitosan 4%, collagen powder 0.3%, glycerol 15%, triclosan 0.3%, azone 10%, and the rest is deionized water.
[0063] Preparation:
[0064] 1) Weigh 40g of carboxymethyl chitosan, add it into 350g of deionized water and stir until completely dissolved to form liquid A, and leave it in place;
[0065] 2) Weigh 3g of collagen powder, add it into 200g of deionized water and stir until completely dissolved to form liquid B, keep it;
[0066] 3) Weigh 100 g of azone and 3 g of triclosan successively, dissolve them in 150 g of glycerol and mix them uniformly to form liquid C;
[0067] 4) Pour liquids B and C into liquid A in turn and mix evenly to form mixed liquid D, then add the remaining deionized water and stir well until they are evenly mixed;
[0068] 5) Filling.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com