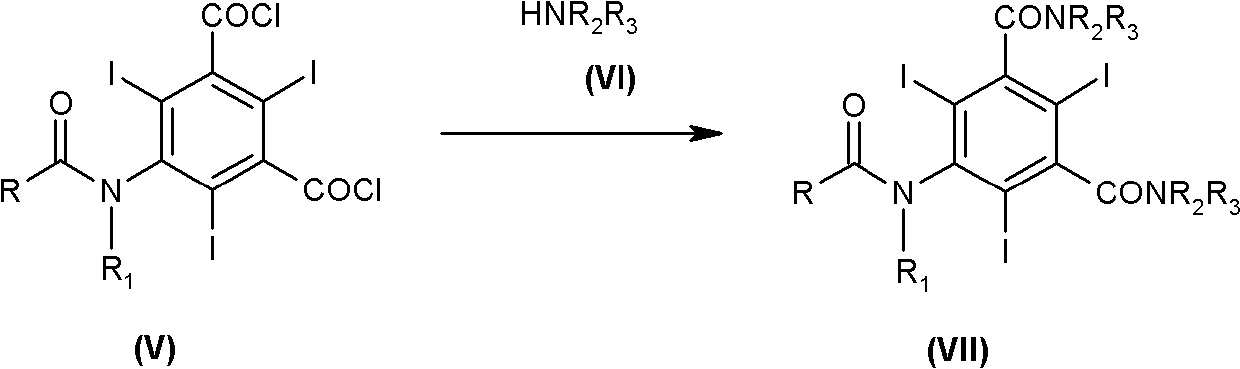
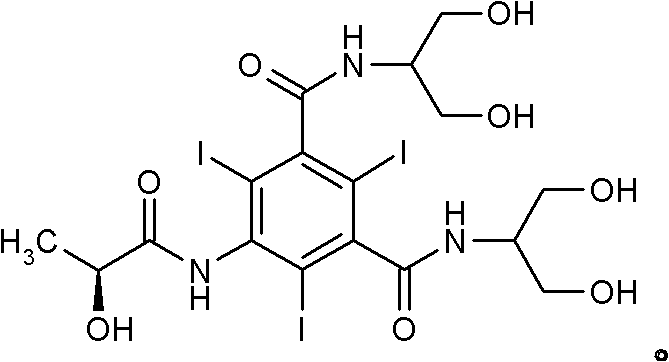
Process for the preparation of iodinated contrast agent
A technology of compounds and hydroxides, applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve problems such as impurity characteristics that damage yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Compounds of formula (III) are prepared starting from isolated compound (II) in the presence of calcium hydroxide.
[0081] Calcium hydroxide (12.8 g, 0.173 mol) was slowly added to a solution of compound (II) (120 g, 0.169 mol) in 305 g of DMA with stirring and keeping the temperature below 25°C.
[0082] Further, to the reaction mixture, a solution of 2-amino-1,3-propanediol in DMA (133 g, 28% w / w, 0.406 mol) was added dropwise over a period of about 45 minutes. The mixture was kept at about 30°C for 10 hours until the reaction was complete.
[0083] According to the procedure of Example 3 below, the crude reaction mass containing the derivative of formula (III) can be purified and hydrolyzed.
[0084] HPLC profile of the mixture after treating the sample with NaOH:
[0085] Iopamidol (IV): 97.9%;
[0086] F-Impurities: 0.2%.
Embodiment 2
[0088] Compounds of formula (III) are prepared starting from isolated compound (II) in the presence of sodium hydroxide.
[0089] Sodium hydroxide (13.9 g, 0.346 mol) was slowly added to a solution of compound (II) (120 g, 0.169 mol) in 305 g of DMA with stirring and keeping the temperature below 25°C.
[0090] Further, a solution of 2-amino-1,3-propanediol in DMA (133 g, 28% w / w, 0.406 mol) was added dropwise to the reaction mixture over about 45 minutes. The mixture was maintained at about 30°C for 10 hours until the reaction was complete.
[0091] According to the procedure of Example 3 below, the crude reaction mass containing the derivative of formula (III) can be purified and hydrolyzed.
[0092] HPLC profile of the mixture after treating the sample with NaOH:
[0093] Iopamidol (IV): 97.4%;
[0094] F-impurity: 0.3%.
Embodiment 3
[0096] Iopamidol (IV) is prepared starting from the isolated compound (II) in the presence of calcium hydroxide.
[0097] A solution of 2-amino-1,3-propanediol in DMA (610 g, 28% w / w, 1.87 mol) was added to compound (II) (600 g, 0.845 mol) in DMAC under stirring for about 45 minutes. (1510g) in solution.
[0098] Then, calcium hydroxide (70.0 g, 0.945 mol) was slowly added to the reaction mixture, keeping the temperature below 30°C. The mixture was further maintained at about 30° C. for 10 hours until the reaction was completed.
[0099] The crude reaction mixture was then distilled under vacuum (95 °C, 10 mbar, 7.5 mmHg) to remove most of the solvent until a viscous residue was obtained. The hot residue was then treated with deionized water (1455 g) and the pH was adjusted to 1.7 by adding hydrochloric acid (33 g, 34% w / w).
[0100] The resulting solution was passed through 1500 mL of Na + form of strong cation exchange resin (Dowex C350 from DOW TM ) to remove Ca 2+ io...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com