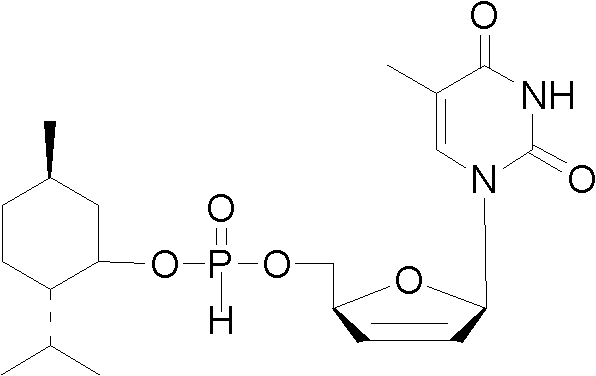
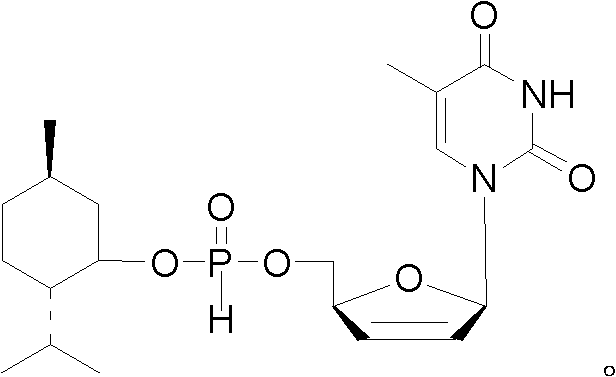
d4T-5'-hydro-ortho-phosphorous acid menthol ester and preparation method and application thereof
A technology of hydrophosphite and menthyl ester, which is applied in chemical instruments and methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as inconvenient operation, achieve short reaction time, mild reaction conditions, and short reaction steps little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
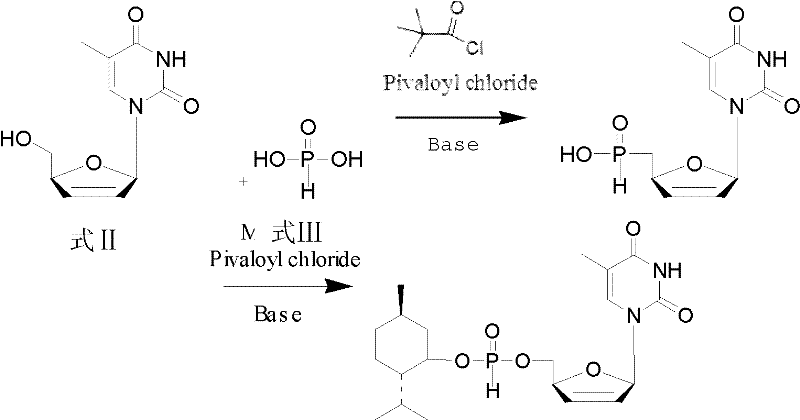
[0030] d4T-5'-menthyl hydrogen phosphite in the present invention is specifically synthesized and prepared by the following method:
[0031] 1) Under the protection of nitrogen, dissolve 2.24g (10mmol) d4T in 50mL of pyridine, then add 1.1eq of phosphorous acid, stir at 25°C to dissolve; under the protection of nitrogen, dissolve 11eq of pivaloyl chloride with 5mL of dichloromethane, Add dropwise to the above reaction solution under stirring, and stir at 25° C. for 5 min after the dropwise addition is complete.
[0032] 2) Under nitrogen protection, 1.2eq of menthol was dissolved in 5mL of dichloromethane, and added dropwise to the reaction solution while stirring.
[0033] 3) Under nitrogen protection, dissolve 1.5eq of pivaloyl chloride in 5mL of dichloromethane, add dropwise to the above reaction solution with stirring, and stir at 25°C for 10min.
[0034] 4) After the reaction, the solid was removed by filtration, and the filtrate was dissolved in dichloromethane after re...
Embodiment 2d4
[0036] Example 2d 4T-5'-menthyl hydrogen phosphite is tested for the anti-HIV-1IIIB virus activity of MT4 cells
[0037] MT4 cell assay d4T-5'-menthyl hydrogen phosphite sample anti-HIV activity assay method is as follows:
[0038] MT-4 cells were seeded in 96-well plates at a cell concentration of 1×10 5 cells / ml, 50 μl per well. Add 100, 10, 1, 0.1, 0.01, 0.001, 0.0001, 0.00001 ng / ml d4T-5′-menthyl hydrogen phosphite 50 μl in different wells, and then add 100 μl HIV-1 virus (100 TCID 50 ). A positive control (cell+AZT+virus), a virus control (cell+virus+culture solution) and a cell control (cell+culture solution) were set up, and the experiment was repeated 3 times. Calculate the half inhibitory concentration IC of the drug against the virus by the Reed-Muench method 50 .
[0039] The experimental results showed that d4T-5′-menthyl hydrogen phosphite had antiviral activity against MT4 / HIV-1IIIB, IC 50 It is 0.48nM, the activity is higher than the positive control drug ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com