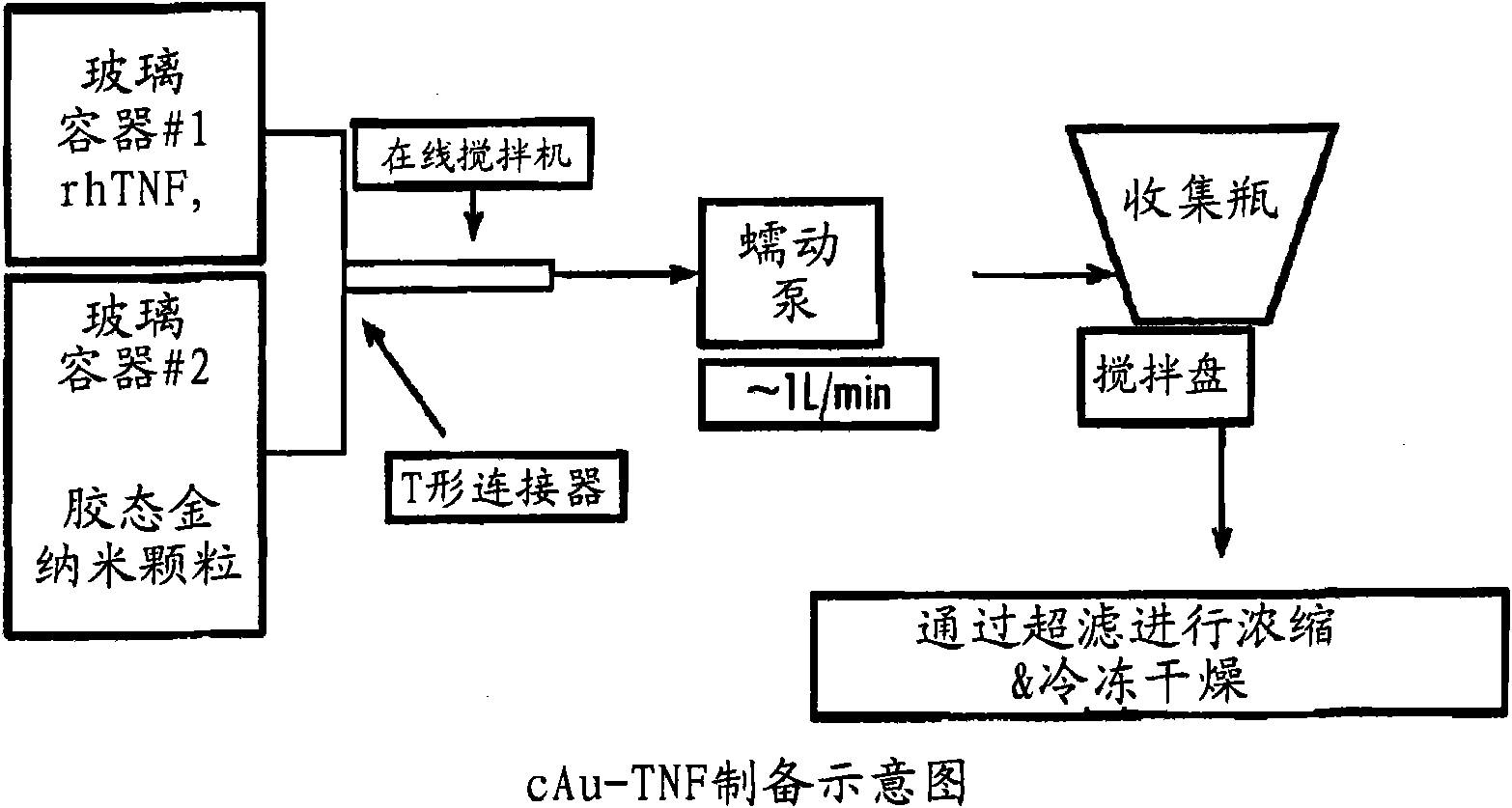
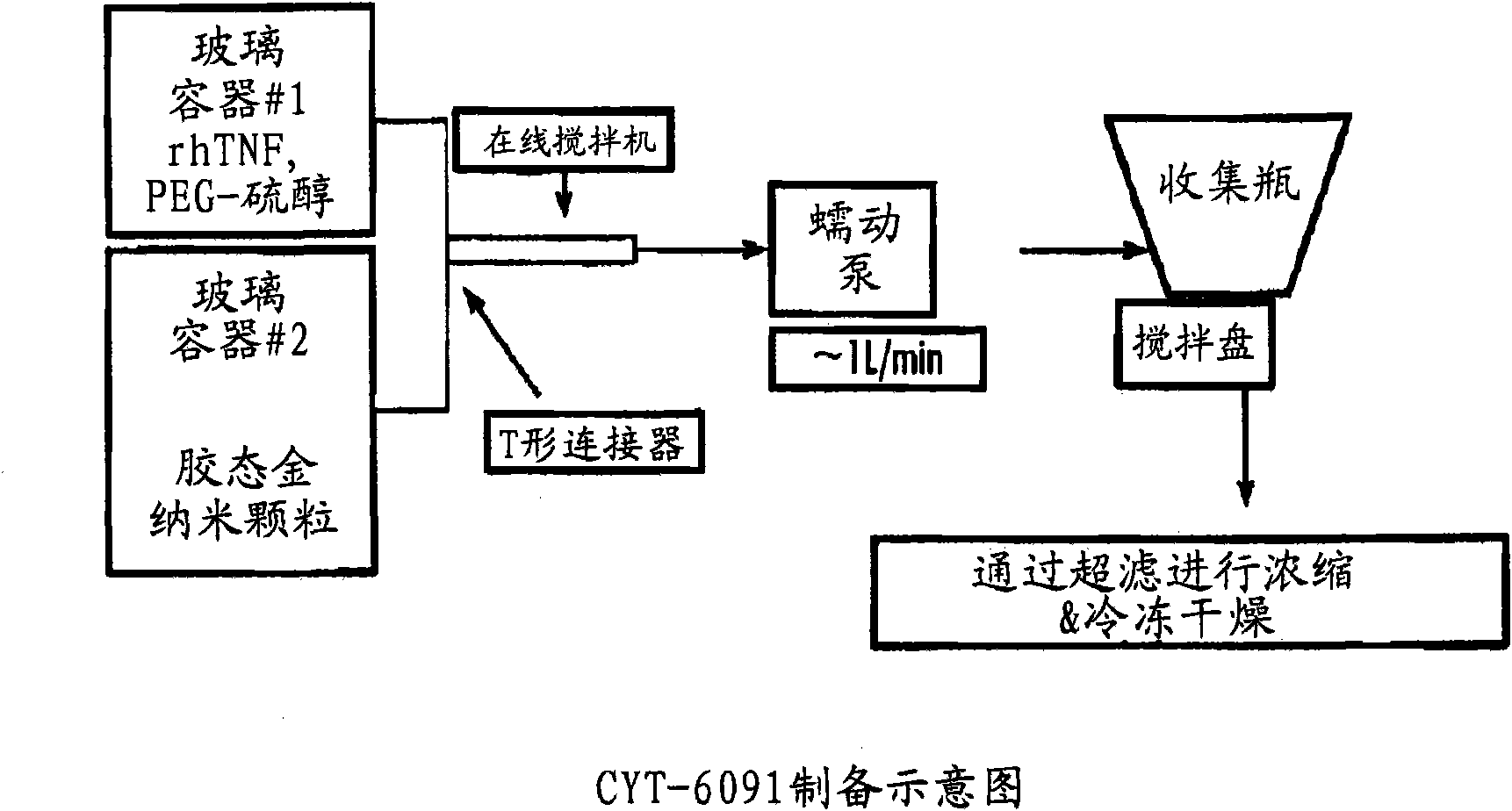
Nanotherapeutic colloidal metal compositions and methods
A technology of colloidal metals and compositions, applied in the direction of hybrid methods, nano-medicines, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
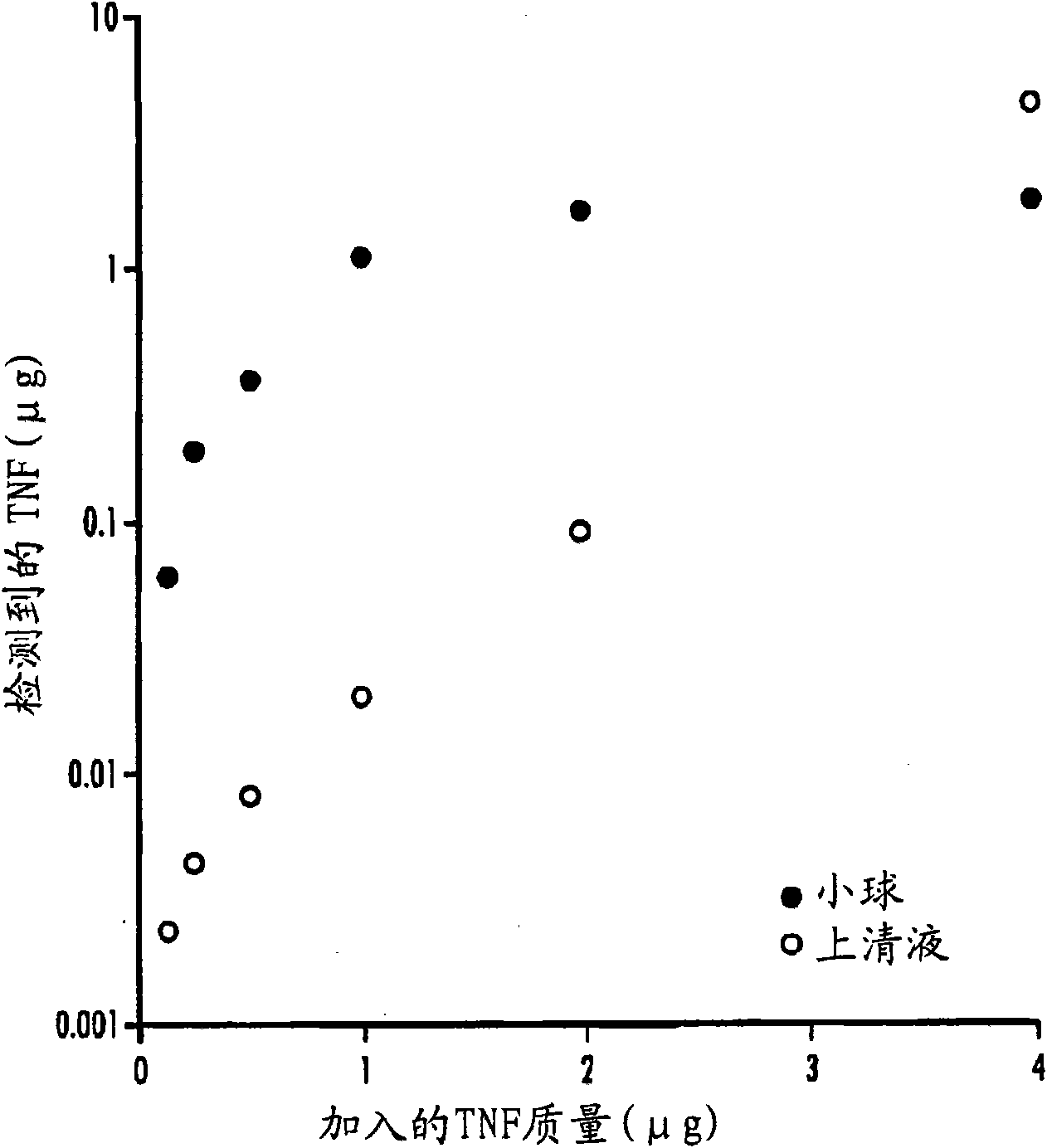
Examples
Embodiment 1
[0162] Preparation of colloidal gold sol
[0163] Chlorauric acid (Au +3 ;HAuCl 4 ) reduced to neutral gold (Au 0 ) to prepare colloidal gold. The method described by Horisberger (1979) is suitable for the preparation of 34 nm colloidal gold particles. This method provides a simple and scalable procedure for preparing colloidal gold. In short, in deionized H 2 O(DIH 2 4% gold chloride solution (23.03% stock solution, dmc 2 , South Plainfield, NJ) and 1% sodium citrate solution (wt / wt; J.T. Baker Company; Paris, KY). Add 3.75 ml of gold chloride solution to 1.5 L of DIH 2 O middle. The solution was stirred vigorously and brought to a boil at reflux. Formation of 34 nm colloidal gold particles was initiated by adding 60 ml of sodium citrate. The solution was continuously boiled and stirred during the entire process of particle formation and growth as described below.
[0164] The addition of sodium citrate to gold chloride initiates a series of reduction reactions ch...
Embodiment 2
[0169] metal source
[0170] Experiments were performed to see if the source of the starting gold reactants that formed the colloidal structure affected the composition of the colloidal gold. Gold chloride was purchased from two different commercial sources: Degussa Metals Catalysts Cerdec (dmc 2 ) and Sigma Chemical Company. The two gold preparations were analyzed for the presence of contaminating metals and other substances. The results of these studies are listed in Table II. Although the gold concentration in each formulation was within the reported values, it is clear that the Sigma formulation contained higher levels of Mg, Ca and Fe.
[0171] Table II. Purity of gold chloride salts used to produce colloidal gold
[0172] element
dmc 2
Sigma
Na
<25ppm
>21ppm
Mg
<25ppm
>60ppm
[0173] Ca
<25ppm
>60ppm
Fe
<25ppm
>60ppm
[0174] TEM of the particles further showed that the...
Embodiment 3
[0177] Using Sigma and dmc 2 Preparation of colloidal gold sol by gold chloride
[0178] The above data indicate that from dmc 2 Gold Chloride contains lower levels of polluting elements. To determine the effect of these two qualitatively different sources of gold chloride, colloidal gold sols were prepared using the two different sources of the salt. The method used to create the colloidal gold particles was as originally described by Horisberger and in Example 1. In short, using from dmc 2 A 4% solution of gold chloride (in water) was prepared with the Sigma stock formulation. 3.75 ml of each solution was added to separate flasks each containing 1.5 L of water. The solution was brought to a rolling boil and kept boiling at reflux with vigorous stirring. 22.5 ml of a 1% sodium citrate solution was added to each flask. The solutions in both flasks were kept boiling until the already described process of colloidal gold formation was complete, marked by a change in gold c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com