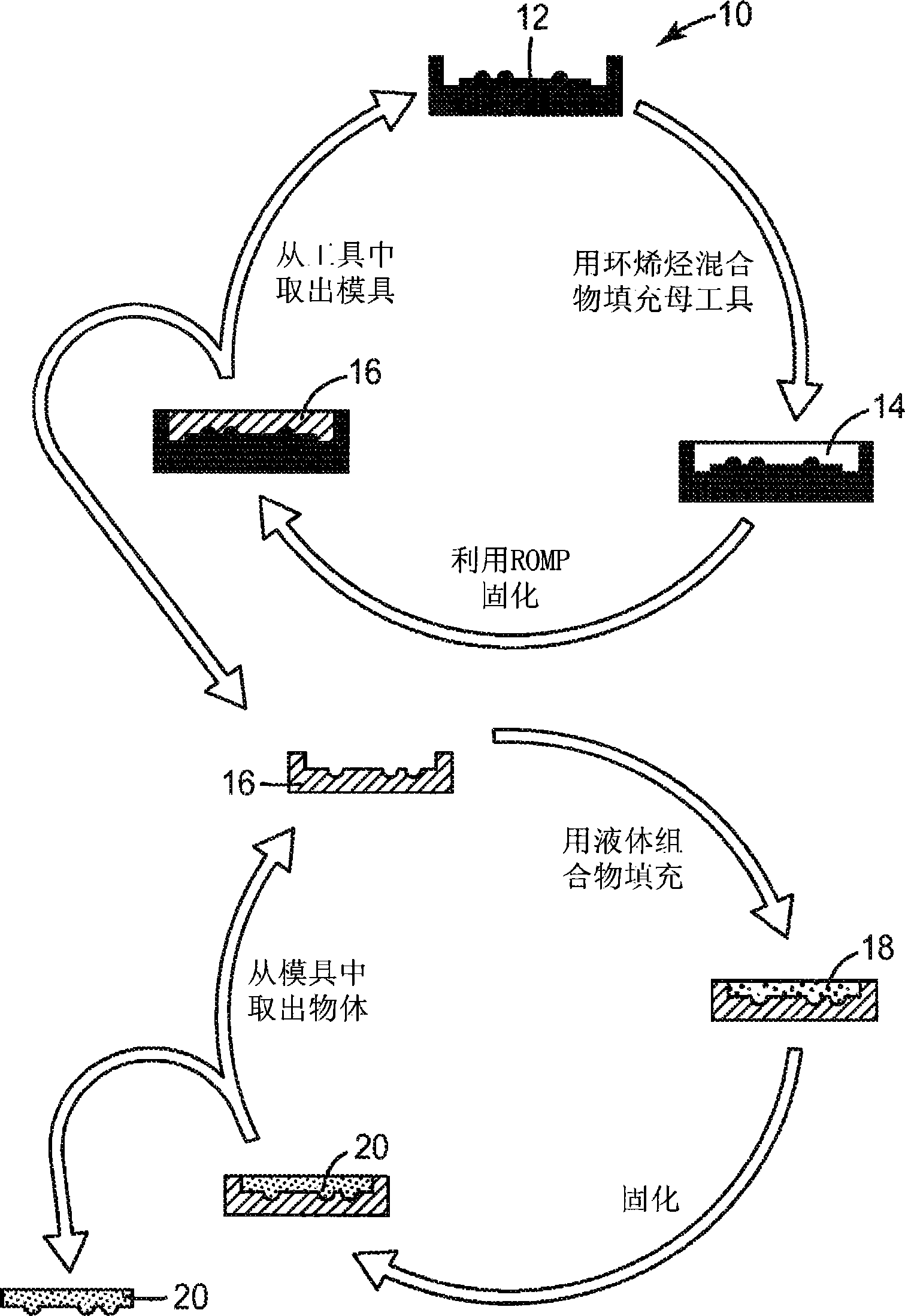
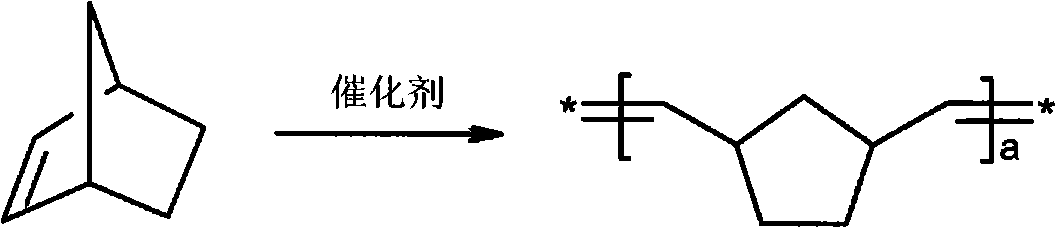
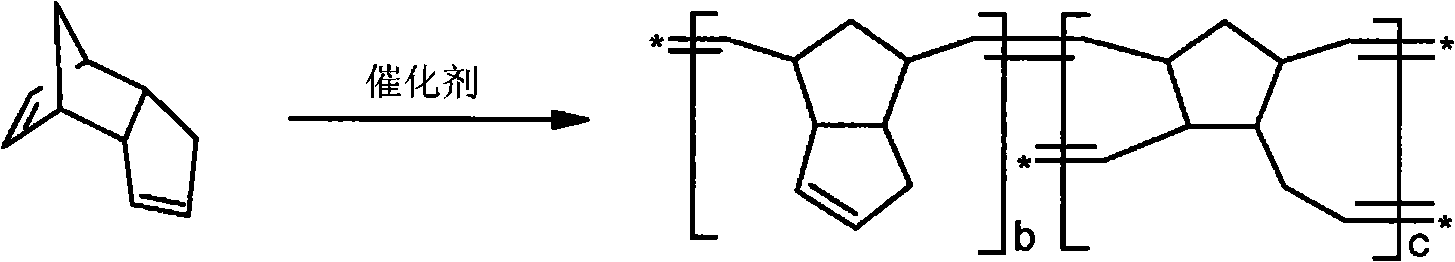
Polymeric molds and articles made therefrom
A polymer and mold technology, applied in the field of polymer molds, can solve the problems of difficult and time-consuming demolding of molded products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0057] All materials were obtained from Aldrich Chemicals (Milwaukee, WI) unless otherwise specified.
[0058] testing method
[0059] Tensile strength (ASTM D638)
[0060] The 1 / 8 inch thick samples were cut into dog bone shaped samples having the dimensions specified in ASTM D638 (Type IV). Tensile strength was measured by testing samples to failure on a Sintech load frame with an initial grip spacing of 3.5 inches and a test speed of 20 inches / minute. To measure elongation, dog bone shaped samples were tested with an initial grip spacing of 1.25 inches and a test speed of 20 inches / minute. Elongation is based on the spacing of the clamps. Marks were added to the samples to identify any slippage in the fixture during the test and any observed slippage was taken into account after the test.
[0061] Tear Strength (ASTM D624)
[0062] Die B was used to cut 1 / 8 inch thick samples into crescent-shaped tear samples as described in ASTM D624. The sample was also notche...
preparation example 1
[0064] Preparation example 1. MeFBSE-norbornene (fluorinated monomer 1)
[0065] Will C 4 f 9 N(CH 3 )CH 2 CH 2 OC(O)CH=CH 2 [121.78 g, 0.296 mol, prepared as described in Parts A and B of Example 2 of WO 01 / 30873A1 (Savu et al.)], cyclopentadiene (23.36 g, 0.353 mol, freshly prepared from dicyclopentadiene) and chloroform (120 mL) of the mixture was mixed at room temperature for 10 minutes. The solution was then heated to 55°C for 20 hours. The mixture was then placed under vacuum to remove solvent. The product was obtained as a waxy solid (yield 140.16 g, 97%).
[0066] Preparation 2.5-norbornene-2-(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro-octyl) ester (Fluorinated monomer 2)
[0067] 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl acrylate (25.00 g, 60 mmol) and cyclopentadiene (4.78 g , 72 mmol, freshly prepared from dicyclopentadiene) and the mixture was mixed at room temperature for 2 hours. The solution was then heated to 55°C for 17 hours. The mixture was...
preparation example 3
[0068] Preparation Example 3: Preparation of W Catalyst Solution
[0069] Charge an oven-dried 1 L flask with WCl under nitrogen 6(4.00 g, 0.010 mol), nonylphenol (2.24 g, 0.010 mol) and about 100 ml of anhydrous toluene. The mixture was stirred for four hours while purging with nitrogen. Anhydrous dicyclopentadiene (DCPD, 500 mL, 3.67 mol) and 2,4-pentanedione (2.0 g, 0.020 mol) were then added. The solution was placed under vacuum and stirred at 40°C for 2.5 hours to remove the toluene. Anhydrous DCPD was added to bring the total volume to 500 mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| friction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com