Mixture of hyaluronic acid for treating and preventing inflammatory bowel disease
A technology for inflammatory bowel disease and hyaluronic acid, applied in the direction of drug combinations, organic active ingredients, medical preparations containing active ingredients, etc., can solve problems such as slow degradation speed, inconvenient use for patients, and inability to maintain curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1-1: Summary
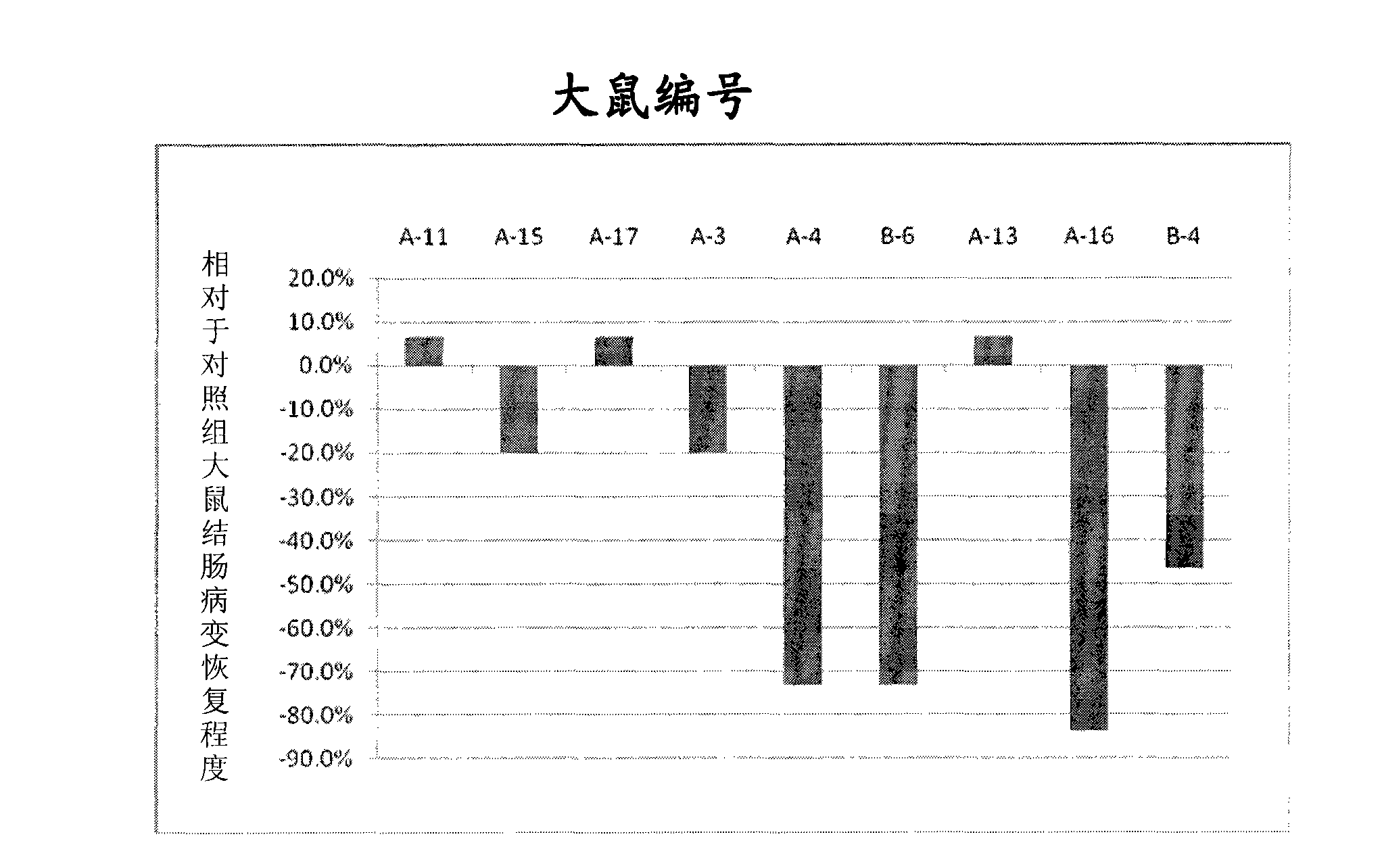
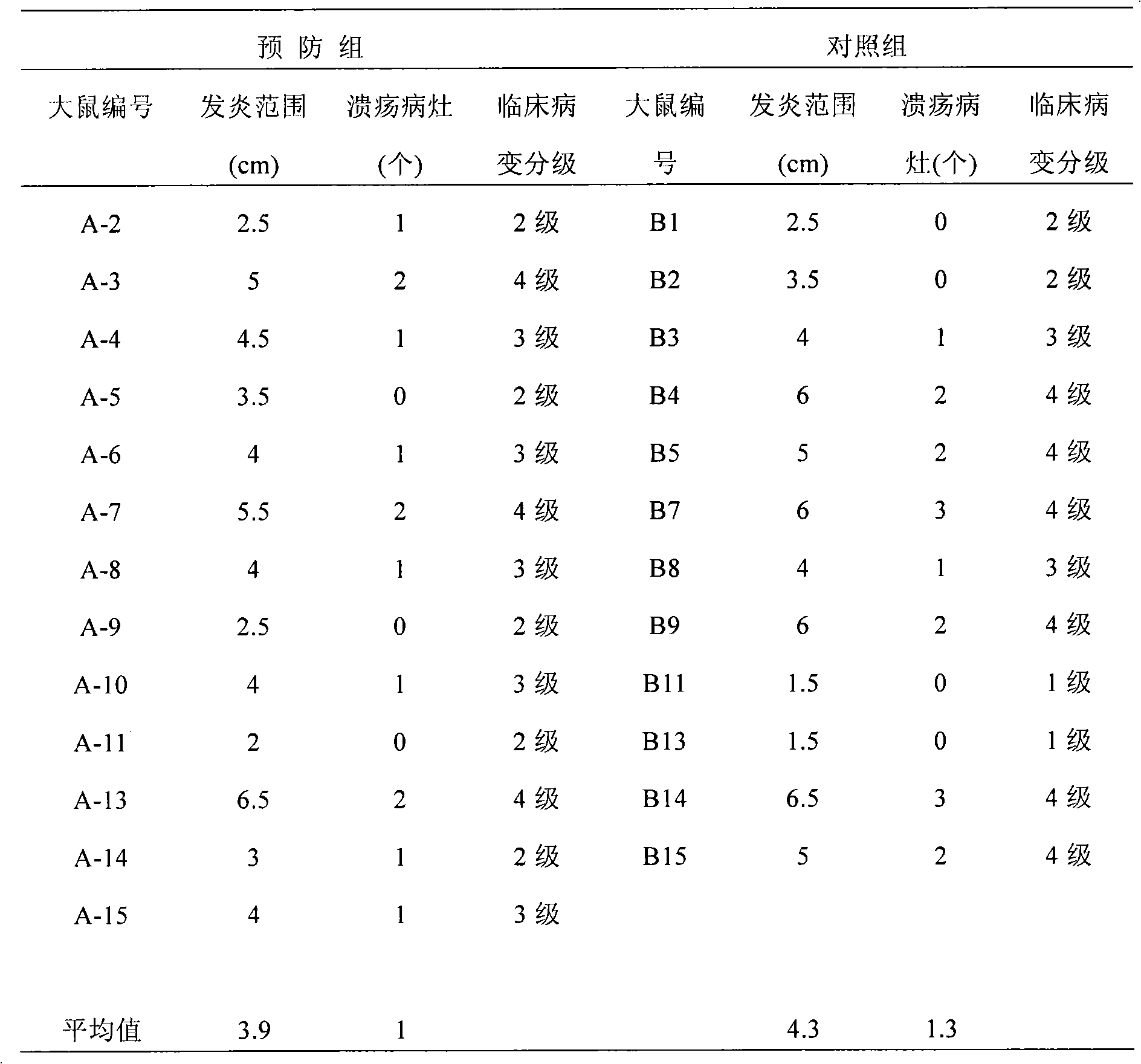
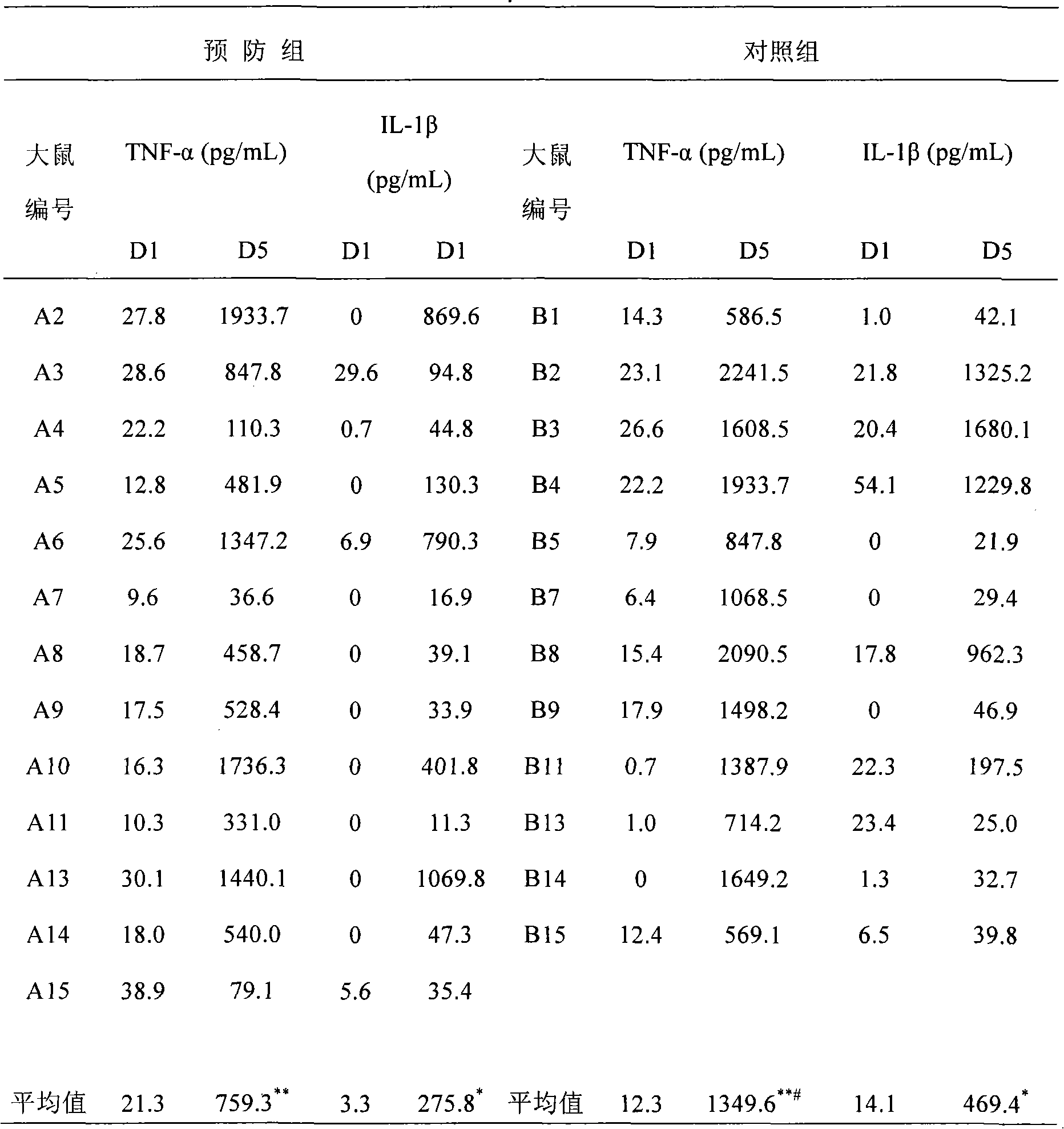
[0036] This test uses the animal model of inflammatory bowel disease to evaluate the efficacy of the sample IBD98 in the animal body. The rats in the prevention group were given IBD98 through the anus first, and then administered trinitrobenzenesulfonic acid (TNBS) to induce enteritis lesions, and then administered through the anus for 3 consecutive days For the sample IBD98, the control group was only given TNBS to induce enteritis lesions, and observed for 3 days. All rats were sacrificed on the 4th day after TNBS administration to examine the clinical lesions, and blood was collected before administration and at the time of sacrifice to detect inflammatory indicators. The results showed that the average range of colonic inflammation in the prevention group and the control group was 3.9 cm and 4.3 cm, respectively, and there was no significant difference between the two. There were significant differences in the concentrations of TNF-α (Tumor Necrosis F...
Embodiment 2
[0064] 2-1: Summary
[0065] This test uses the animal model of inflammatory bowel disease to evaluate the efficacy of the sample IBD98 in the animal body. The experimental rats are first administered with trinitrobenzenesulfonic acid (TNBS) through the anus to induce enteritis lesions, and then three days later, the samples IBD98 are administered through the anus. (Treatment group) and buffered phosphate saline (PBS) (control group) were treated continuously for seven days, and then the rats were sacrificed to examine the clinical lesions. The results showed that the colonic inflammation range of the rats in the treatment group injected with the sample IBD98 was about 1.25 cm on average, The average range of inflammation in the colon of rats administered with buffered phosphate saline in the control group was about 1.875 cm, and the relative degree of recovery compared with the treatment group and the control group was 33%, showing that the use of IBD98 to treat inflammatory b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com