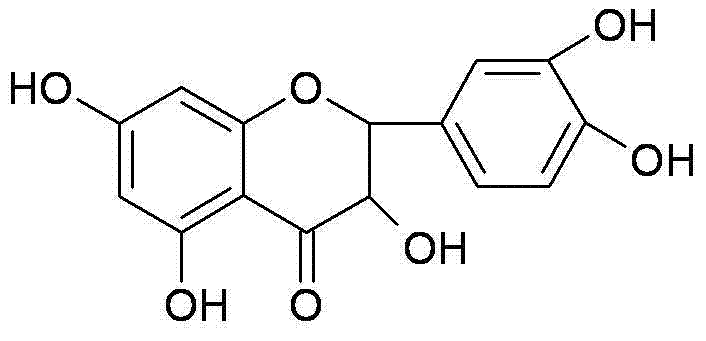
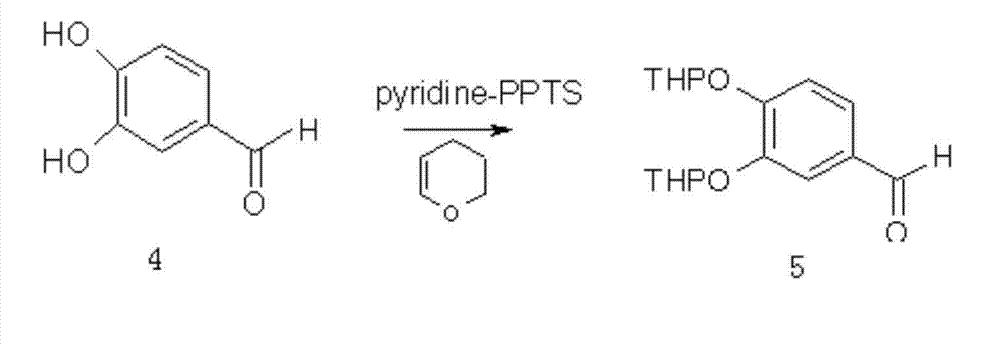
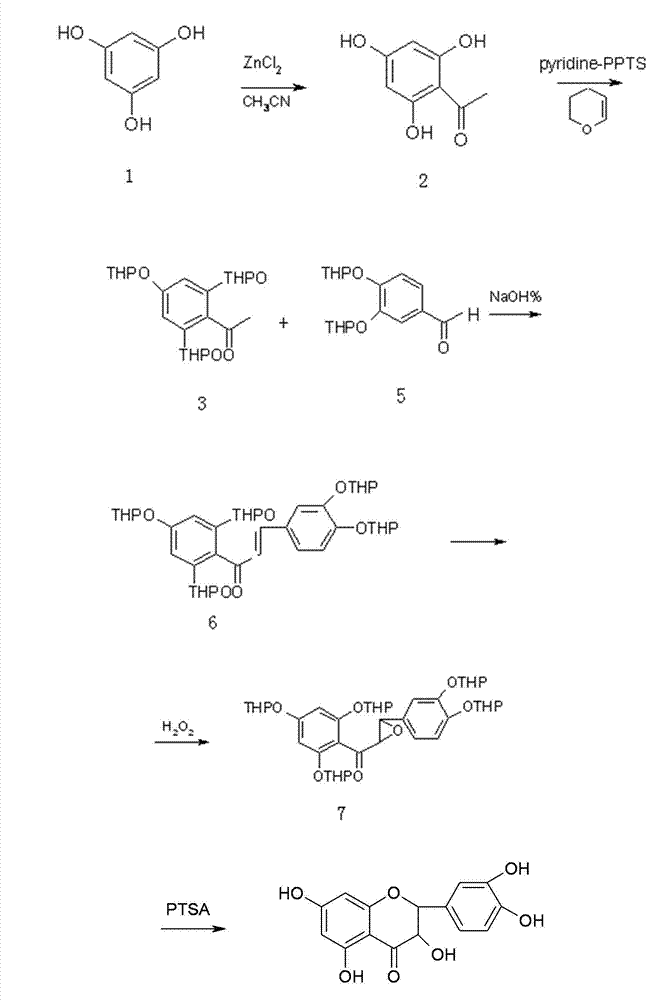
Synthesis method of dihydroquercetin
A technology of dihydroquercetin and a synthesis method, which is applied in the field of synthesis of dihydroquercetin, can solve the problems of high production cost of dihydroquercetin, unsuitability for large-scale industrial production, etc., and achieve shortened production time, easy Effect of industrialized production and reduction of production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The synthetic method of this dihydroquercetin comprises the following steps:
[0038] 1] Preparation of 2,4,6-trihydroxyacetophenone pyryl ether
[0039] 1.1] Preparation of 2,4,6-trihydroxyacetophenone
[0040] Take anhydrous phloroglucinol and acetonitrile, and prepare 2,4,6-trihydroxyacetophenone through Hoesch reaction according to the dosage of 1 mol: 0.5~2.0 mol;
[0041] 1.2] Preparation of 2,4,6-trihydroxyacetophenone tripyranyl ether
[0042] Dissolve the 2,4,6-trihydroxyacetophenone prepared in step 1.1 into a solvent for reaction, add 3,4-dihydropyran and a catalyst during the reaction, and the reaction temperature is 20-80°C. After the reaction is completed Generate 2,4,6-trihydroxyacetophenone dipyranyl ether; the feed ratio of the 2,4,6-trihydroxyacetophenone, 3,4-dihydropyran and catalyst is 1 mol:2.0 ~6.0mol: 0.2~0.6mol; the volume of the solvent is 5~50 times the mass of 2,4,6-trihydroxyacetophenone; the end point of the reaction is detected by TLC; 2,...
Embodiment 1
[0053] 1] Preparation of 2,4,6-trihydroxyacetophenone pyryl ether
[0054] 1.1] Preparation of 2,4,6-trihydroxyacetophenone
[0055] In a 500ml three-necked reaction flask, add 150ml of isopropyl ether, anhydrous ZnCl 2 16g, stir and disperse, then add 25.2g of anhydrous phloroglucinol and 12.3g of acetonitrile, and continue stirring; the low temperature reactor controls the temperature at 0-2°C. Infuse saturated HCl gas (produced by adding concentrated hydrochloric acid to concentrated sulfuric acid dropwise), continuously ventilate for 2 days, then place at room temperature for 3 days, the reaction stops; pour out isopropyl ether (it can be used as a solvent next time), and in the Add 200ml of water and reflux for 2h, cool to obtain 32.3g of yellow solid with a yield of 87%;
[0056] 1.2] Preparation of 2,4,6-trihydroxyacetophenone tripyranyl ether
[0057] Add 18.6g of 2,4,6-trihydroxyacetophenone and 7.5gmol of pyridinium p-toluenesulfonate into a 500ml three-necked rea...
Embodiment 2
[0067] 1] Preparation of 2,4,6-trihydroxyacetophenone pyryl ether
[0068] 1.1] Preparation of 2,4,6-trihydroxyacetophenone
[0069] In a 500ml three-necked reaction flask, add 150ml of isopropyl ether, anhydrous ZnCl 2 20.4g, stirred and dispersed, then added 25.2g of anhydrous phloroglucinol and 12.3g of acetonitrile, and continued to stir. The temperature of the low temperature reactor is controlled at 0-2°C; saturated HCl gas (generated by adding concentrated hydrochloric acid to concentrated sulfuric acid) is passed through, continuously ventilated for 2 days, and then left at room temperature for 3 days, the reaction is stopped; pour out isopropyl ether ( It can be used as a solvent next time), add 200ml of water to the residue, reflux for 2h, cool down, and obtain 31.6g of yellow solid with a yield of 85%;
[0070] 1.2] Preparation of 2,4,6-trihydroxyacetophenone pyryl ether
[0071] Add 18.6g of 2,4,6-trihydroxyacetophenone and 7.5g of pyridinium p-toluenesulfonate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com