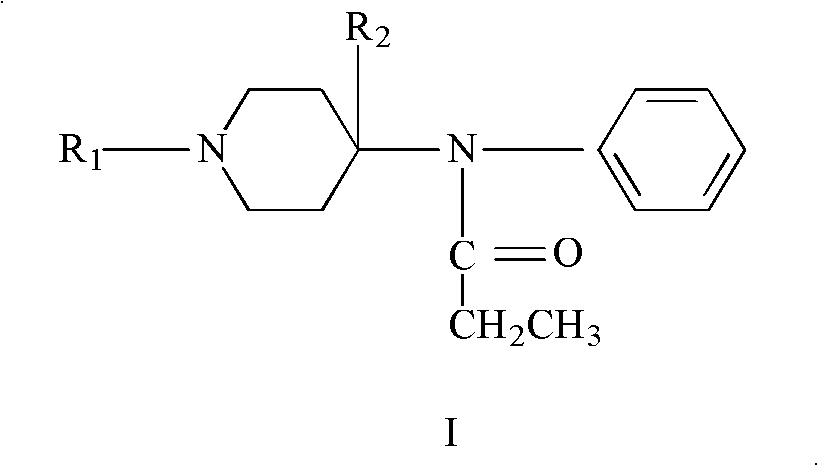
Refining method of 4-phenylaminopiperidine analgesic
A technology of anilinopiperidine and a purification method, applied in directions such as organic chemistry, can solve problems such as being unsuitable for industrialized production, time-consuming, labor-intensive cost, low acid salt yield, etc., achieving simple and easy-to-obtain reagents, reducing production costs, and techniques simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
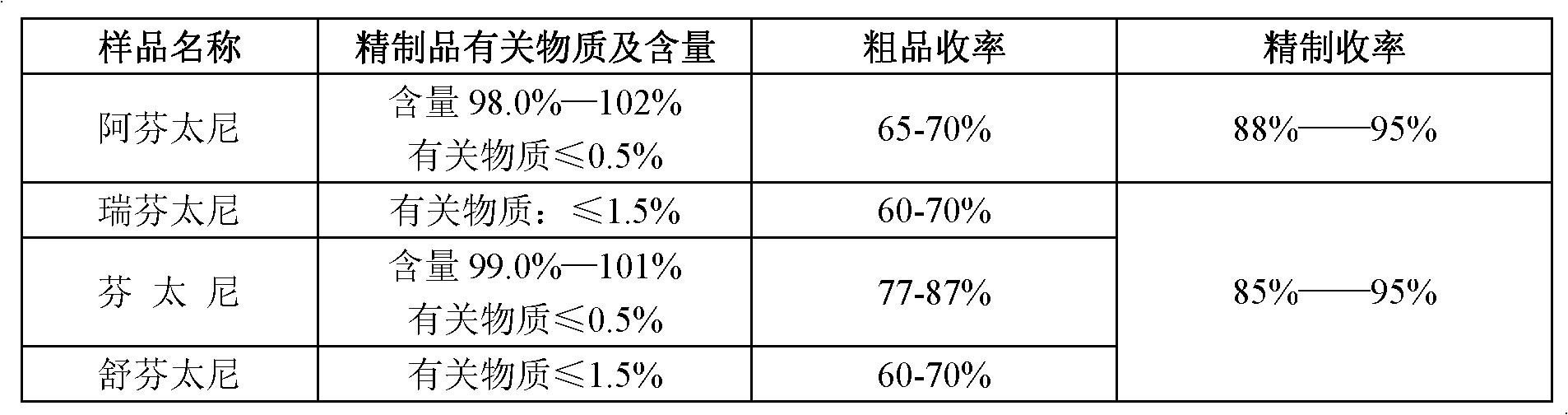
[0036] Mix 10 g of crude fentanyl, 6 g of methanesulfonic acid, and 30 ml of methanol to obtain fentanyl mesylate.
[0037] Fentanyl mesylate is added to ethanol at 1:3 by weight (g): volume (ml), stirred and heated to reflux, adding 0.05 times the weight of fentanyl mesylate for decolorization with activated carbon, after filtration The filtrate was cooled and crystallized, and filtered to obtain the refined product of fentanyl mesylate.
[0038] Fentanyl mesylate was dissolved in water according to weight (g): volume (ml) = 1:20, and alkali was added to neutralize to pH 10, and a white solid was precipitated to obtain fentanyl base. Add citric acid to the fentanyl base solution (the solvent is a lower aliphatic alcohol below C7, the same below), to obtain 12.4 g of fentanyl citrate, with a crude yield of 79%.
[0039] Dissolve 12.4 g of fentanyl citrate in 87 ml of ethanol-ether (volume ratio 6:5) mixed solution, heat up to reflux, add 0.03 times of activated carbon for dec...
Embodiment 2
[0041] Mix 10 g of crude fentanyl, 5 g of oxalic acid, and 30 ml of methanol to obtain fentanyl oxalate.
[0042] Add fentanyl oxalate to ethanol at a weight (g): volume (ml) ratio of 1:5, stir and raise the temperature to reflux, add activated carbon 0.05 times the weight of fentanyl oxalate for decolorization, filter and crystallize the filtrate by cooling , filtered to obtain refined fentanyl oxalate.
[0043] Add fentanyl oxalate to water according to weight (g): volume (ml) = 1:20 to dissolve, add alkali to neutralize to pH 10, and precipitate white solid to obtain fentanyl base. Add citric acid to the fentanyl base solution to obtain fentanyl citrate, 13.2 g, with a crude yield of 84%. .
[0044] Dissolve 13.2 g of crude fentanyl citrate in 145 ml of methanol-ether (volume ratio 1:0.5) mixed solution, heat up to reflux, add 0.03 times of activated carbon for decolorization and recrystallization, and obtain 12.0 g of refined fentanyl citrate. Yield 90%.
Embodiment 3
[0046] Mix 10 g of crude remifentanil, 5 g of oxalic acid, and 23 ml of ethanol to obtain remifentanil oxalate.
[0047] Add remifentanil oxalate into ethanol at a weight (g): volume (ml) ratio of 1:3, stir and raise the temperature to reflux, add 0.05 times of activated carbon for decolorization, and filter the filtrate to cool and crystallize, and filter to obtain remifentanil Refined oxalate.
[0048] Remifentanil oxalate was added to water to dissolve at weight (g): volume (ml) = 1:13, and alkali was added to neutralize to pH 10, and a white solid was precipitated, which was the base of remifentanil. Concentrated hydrochloric acid was added dropwise to the base solution of remifentanil to obtain 9.0 g of remifentanil hydrochloride, and the yield of the crude product was 63%. .
[0049] Dissolve 9.0 g of remifentanil hydrochloride crude product in 90 ml of methanol-acetone (volume ratio 1:1) mixed solution, raise the temperature to reflux, add 0.03 times of activated carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com