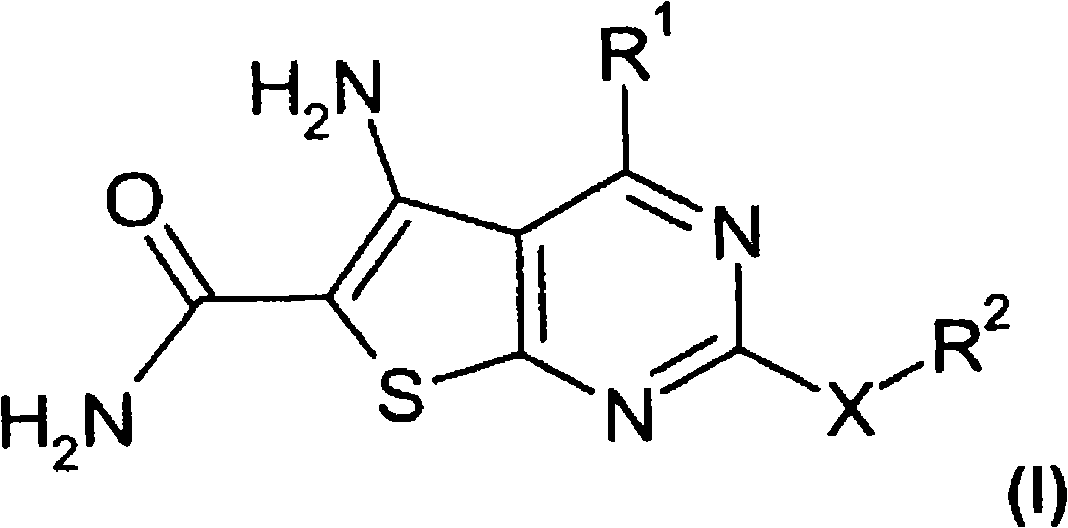
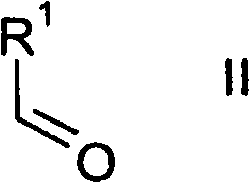
Thienopyrimidines
A technology of pyrimidine and thiophene, which is applied in the field of new compounds, can solve the problems of increased anticancer treatment and increased risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0232] Preparation of 5-amino-2-cyclopropyl-4-(5-methylfuran-2-yl)thieno[2,3-d]pyrimidine-6-carboxamide ("A1")
[0233] Synthetic flow diagram for the synthesis of "A1"
[0234]
[0235] 1.1 In a three-necked flask, 9.1 ml of 5-methyl-2-carboxyfurfuraldehyde ("E1") was dissolved in 70 ml of dichloromethane. Then 8 ml of methyl cyanoacetate and 45 g of alumina were added, and the mixture was stirred at room temperature for 2 h.
[0236] For work-up, the aluminum oxide was filtered off with suction. Flush it well with dichloromethane. The yellow solution was evaporated until only solids remained, yielding 15.3 g of methyl 2-cyano-3-(5-methylfuran-2-yl)acrylate.
[0237] HPLC-MS: [M+H]192
[0238] 1.2 In preparation, 460 mg of elemental sodium was dissolved in 8.0 ml of dry ethanol in a round bottom flask with a drying tube. 3.827 g of methyl 2-cyano-3-(5-methylfuran-2-yl)acrylate and 2.49 g of cyclopropylguanidine (“E2”) hydrochloride were then suspended in 35 ml of a 10...
Embodiment 2
[0268] Preparation of 5-amino-4-furan-2-yl-2-methylthiothieno[2,3-d]pyrimidine-6-carboxamide ("A8")
[0269] 2.1 Combine 13ml of furfural and 13.3ml of methyl cyanoacetate in a flask, add 60g of alumina, and the temperature rises to 53°C during the process. After adding 50 ml of dichloromethane, the reaction mixture was stirred at room temperature for 2 hours. For work-up, the aluminum oxide was filtered off and the filtrate was evaporated, yielding 23.3615 g of methyl 2-cyano-3-furan-2-ylacrylate ("E3").
[0270] HPLC content: 97.7%
[0271] HPLC-MS: [M+H]178
[0272] 2.2 In preparation, 1.3 g of elemental sodium was dissolved in 15 ml of ethanol. 5 g of methyl 2-cyano-3-furan-2-ylacrylate and 5.2 g of thiourea ("E4") were then suspended in 50 ml of butanol in a 250 ml round bottom flask and dissolved sodium ethoxide was added. The suspension was stirred at 110°C for 5.5 hours.
[0273] For work-up, the batch was cooled to room temperature, poured onto ice, adjusted to p...
Embodiment 3-37
[0291] Carrying out the operation of Example 1, but using furan-2-carbaldehyde as "E1" and guanidine as "E2" to give 2,5-diamino-4-furan-2-ylthieno[2,3-d] Pyrimidine-6-carboxamide ("A11") δ 8.0 (d, 1H), 7.3 (d, 1H), 7.2-6.8 (BR, 6H), 6.7 (m, 1H), 4.6 (d, 1H).
[0292]
[0293] Carry out the operation of Example 1, but use guanidine as "E2" to obtain 2,5-diamino-4-(5-methylfuran-2-yl)thieno[2,3-d]pyrimidine-6-methan Amide ("A11a") δ 7.2 (m, 3H), 7.0 (s, 2H), 6.9 (s, 2H), 6.4 (d, 1H), 2.4 (s, 3H).
[0294]
[0295] Carry out the operation of Example 1, but use benzofuran-2-carbaldehyde as "E1" and guanidine as "E2" to obtain 2,5-diamino-4-benzofuran-2-ylthieno[2, 3-d] pyrimidine-6-carboxamide ("A12") δ 7.8(dd, 2H), 7.7(s, 1H), 7.5(t, 1H), 7.4(t, 1H), 7.2(s, 2H ), 7.1(s, 2H), 7.0(s, 2H).
[0296]
[0297] Carrying out the procedure of Example 1 but using benzofuran-2-carbaldehyde as "E1" and pyridine-2-carboxamidine as "E2" gives 5-amino-4-benzofuran-2-yl-2- Pyridin-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com