Tetrahydro thienopyridine derivative for treating
A compound and hydrate technology, applied in the field of tetrahydrothienopyridine derivatives, can solve problems such as plaque rupture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
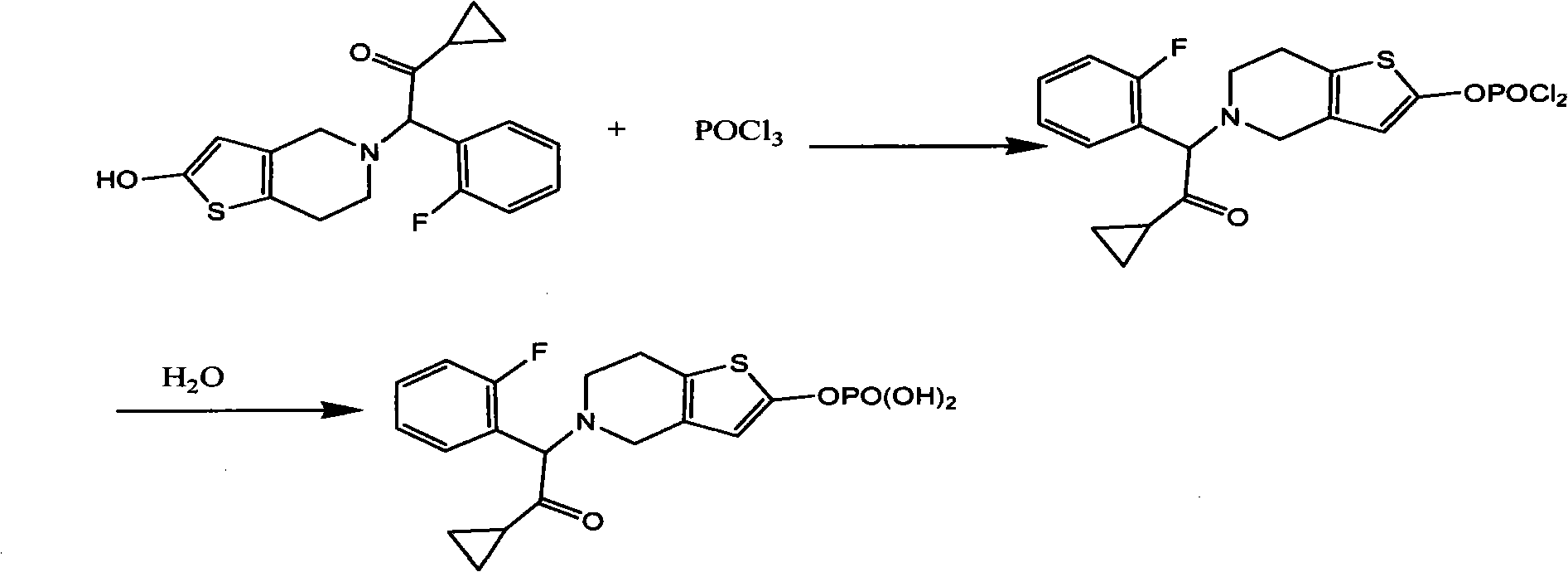
Image
Examples
Embodiment 1
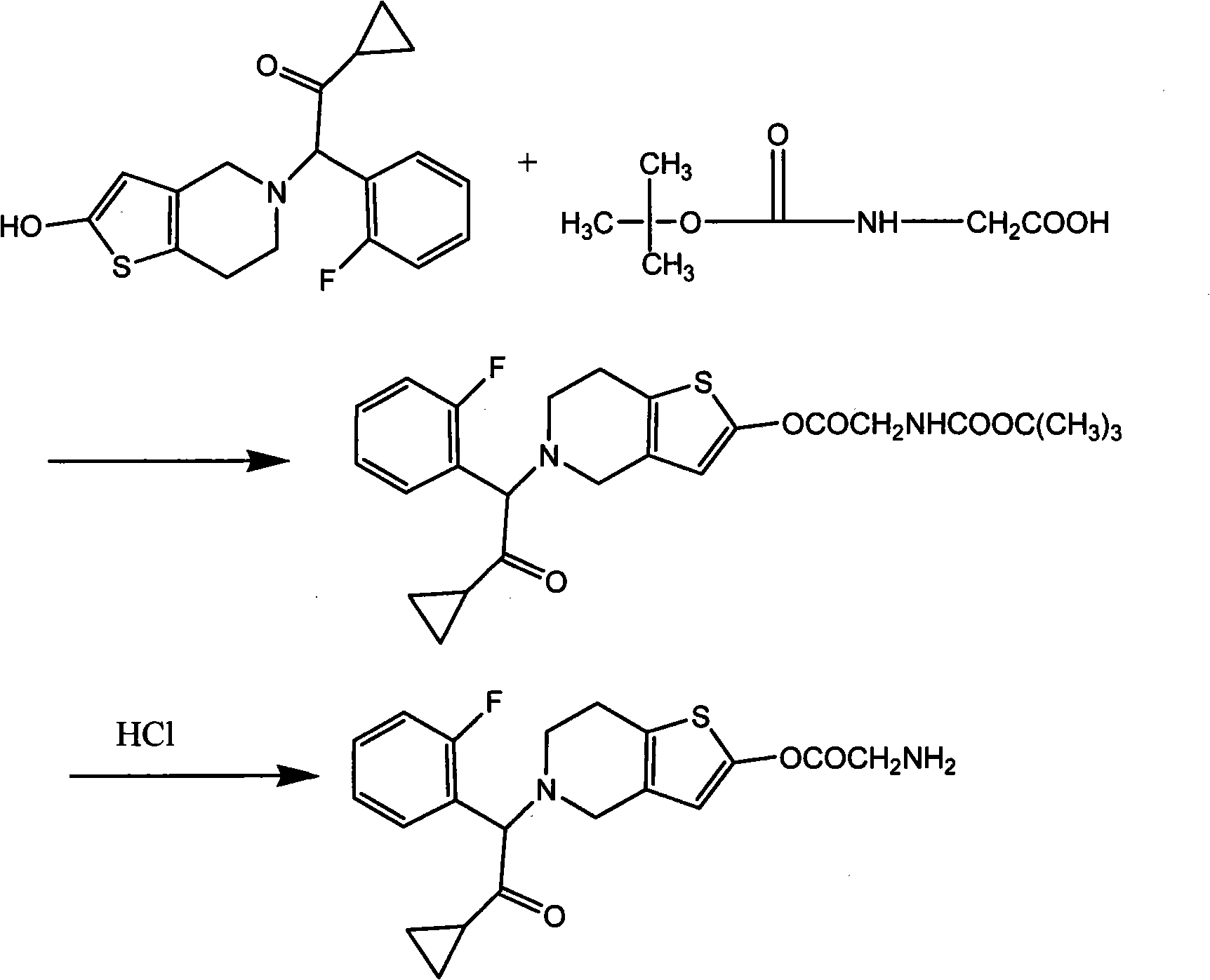
[0021] Embodiment 1: the preparation of compound A
[0022] Reaction equation:
[0023]
[0024] 2-[2-(hydroxyl)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl]-1-cyclopropyl-2-(2-fluorophenyl) 37.3 g (0.1 mol) of ethyl ketone was dissolved in 2000 ml of ethyl acetate, 26.3 g (0.1 mol) of triphenylphosphine and 17.52 g (0.1 mol) of BOC-glycine were added, and diethyl azodicarboxylate was added dropwise under stirring (DEAD) 17.42g (0.1mol) was reacted at room temperature for 5h, filtered, dry HCl gas was passed into the filtrate to saturation, stirred at room temperature for 5h, a large amount of white solid was precipitated, filtered, the filter cake was washed with a small amount of ethyl acetate, and dried to obtain the compound A hydrochloride 13.8g.
[0025] After dissolving 10 g of compound hydrochloride with 100 ml of pure water, slowly add saturated sodium carbonate solution dropwise until the pH is 10 to 10.5, extract three times with 500 ml each time of ethyl acetate,...
Embodiment 2
[0026] Example 2: Preparation of R, S-type isomers of compound A.
[0027] Prepared according to the method of Example 1 except that R-type 2-[2-(hydroxyl)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl]-1-ring Propyl-2-(2-fluorophenyl)ethanone or S-type 2-[2-(hydroxyl)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl] -1-cyclopropyl-2-(2-fluorophenyl)ethanone instead of 2-[2-(hydroxyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)- Base]-1-cyclopropyl-2-(2-fluorophenyl)ethanone.
Embodiment 3
[0028] Example 3: Preparation of Compound B.
[0029] 2-[2-(hydroxyl)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl]-1-cyclopropyl-2-(2-fluorophenyl) 37.3 g (0.1 mol) of ethyl ketone was dissolved in 2000 ml of ethyl acetate, 26.3 g (0.1 mol) of triphenylphosphine and 18.92 g (0.1 mol) of BOC-alanine were added, and diethyl azodicarboxylate was added dropwise under stirring. Ethyl ester (DEAD) 17.42g (0.1mol) was reacted at room temperature for 5h, filtered, dry HCl gas was passed through the filtrate to saturation, stirred at room temperature for 5h, a large amount of white solid was precipitated, filtered, the filter cake was washed with a small amount of ethyl acetate, dried 17.3 g of compound B hydrochloride was obtained.
[0030] After dissolving 10 g of compound B hydrochloride with 80 ml of pure water, slowly add saturated sodium carbonate solution dropwise until the pH is 10 to 11, extract three times with 500 ml of ethyl acetate, combine the extracts, and add anhydrous so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com