Process for preparing high-purity rocuronium
A high-purity technology for rocuronium bromide, which is applied in the production of steroids, bulk chemicals, and muscular system diseases, can solve the problems of increasing the complexity and cost of the production process of rocuronium bromide, and achieve high purity and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
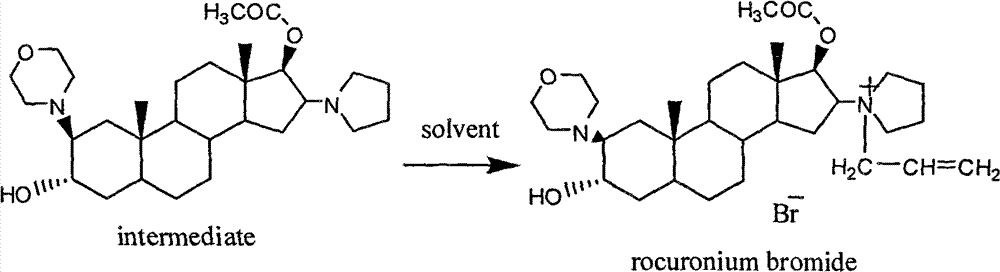
[0024] The preparation of embodiment 1 high-purity rocuronium bromide:
[0025] Add 18 g of 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androst-3α-ol and 17β-acetate into a single-port reaction flask, add the carapace that is crushed to 80-150 mesh Element 2g, anhydrous magnesium sulfate 1g, dichloromethane 180ml and 3-bromopropene 12ml, stirred at room temperature for 8 hours, carried out with thin layer chromatography (TLC) and HPLC (by the condition that EP6.0 detects rocuronium bromide related impurity) ) to monitor the reaction process, and when the ratio of the peak area of the raw material in the HPLC chromatogram is less than 1%, stop the reaction, filter the reaction mixture, and reclaim dichloromethane to obtain an oil. Dissolve the oily matter with 30 ml of dichloromethane, add the resulting solution to 400 ml of ethyl acetate with constant stirring, and stir for 10 minutes. Filter out the solids and put in CO 2 In a supercritical extraction kettle, the temperatu...
Embodiment 2
[0027] The preparation of embodiment 2 high-purity rocuronium bromide:
[0028] Add 18 g of 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androst-3α-ol and 17β-acetate into a single-port reaction flask, add the carapace that is crushed to 80-150 mesh Element 2g, anhydrous magnesium sulfate 1g, dichloromethane 180ml and 3-bromopropene 12ml, stirred at room temperature for 11 hours, carried out with thin-layer chromatography (TLC) and HPLC (by EP6.0 detecting the condition of rocuronium bromide related impurity) ) to monitor the reaction process, when the ratio of the peak area of the raw material in the HPLC chromatogram is less than 0.5%, the reaction is stopped, the reaction mixture is filtered, and the resulting filtrate is directly dropped into CO 2 In the supercritical extraction kettle, the temperature in the kettle is adjusted to 25°C, carbon dioxide is introduced into the kettle, and the pressure adjustment force is 12MP. 12MP, then adjust the temperature to 30°C, and r...
Embodiment 3
[0030] The preparation of embodiment 3 high-purity rocuronium bromide:
[0031] Add 18 g of 2β-(4-morpholinyl)-16β-(1-pyrrolidinyl)-5α-androst-3α-ol and 17β-acetate into a single-port reaction flask, add the carapace that is crushed to 80-150 mesh Element 2g, dichloromethane 180ml and 3-bromopropene 12ml, room temperature, stir 16 hours under nitrogen protection, monitor the reaction progress with thin-layer chromatography (TLC), after the raw material is substantially consumed, the reaction mixture is filtered, then reclaims two Chloromethane was obtained as an oil. Dissolve the oil with a small amount of 30ml of acetonitrile, and add the resulting solution into 400ml of ethyl acetate under constant stirring, and stir for 10 minutes. Filter out the solids and put in CO 2 In a supercritical extraction kettle, the temperature in the kettle is adjusted to 25°C, carbon dioxide is introduced into the kettle, and the pressure is adjusted to 9MP; g
[0032] Detect according to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com