Method for synthesizing N,N'-diphenyl-N-(9,9-dimethyl-2-fluorenyl)-N'-(9',9'-dimethyl-7'-bromo-2'-fluorenyl)-benzidine
A kind of synthetic method, technology of dimethyl, applied in N field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
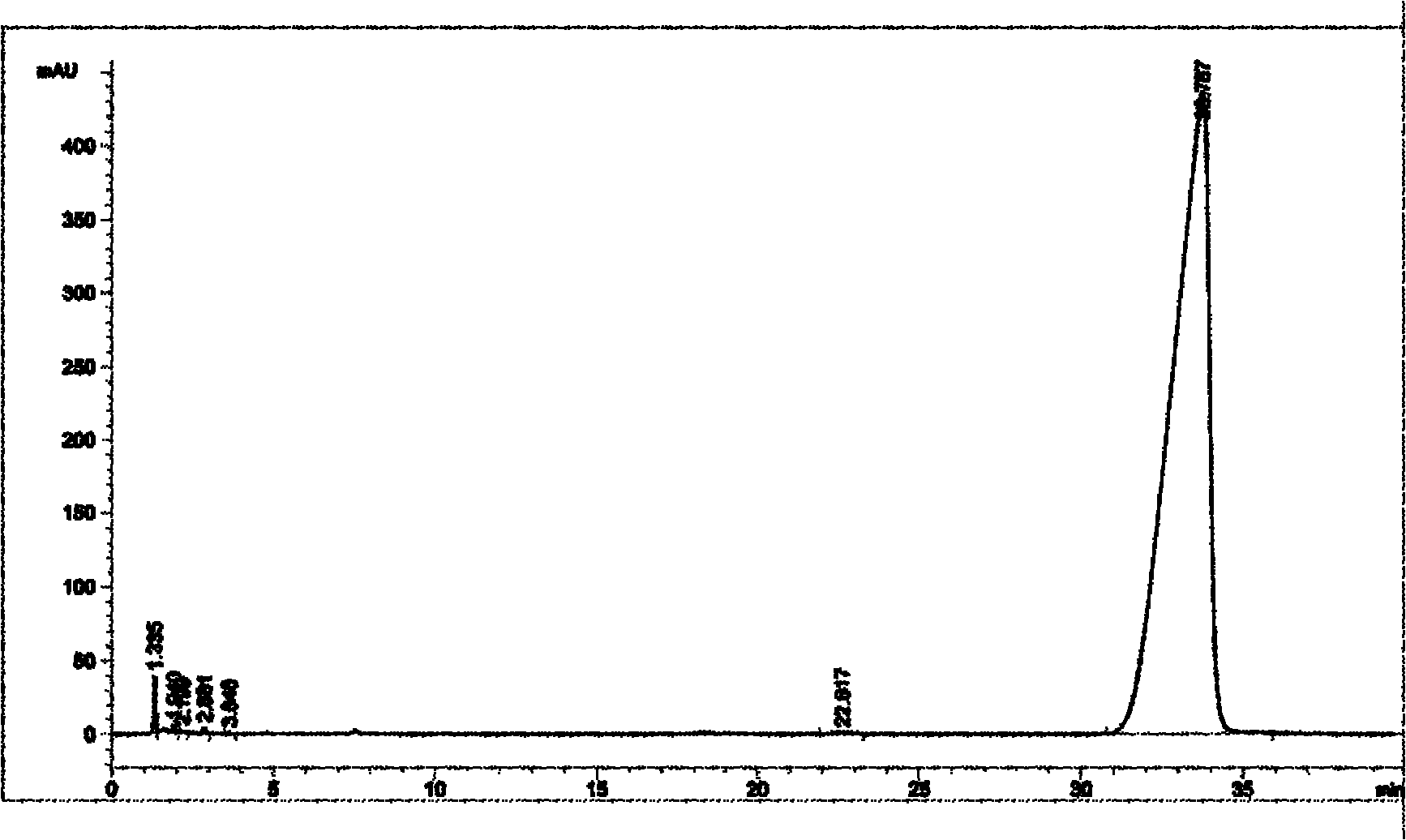
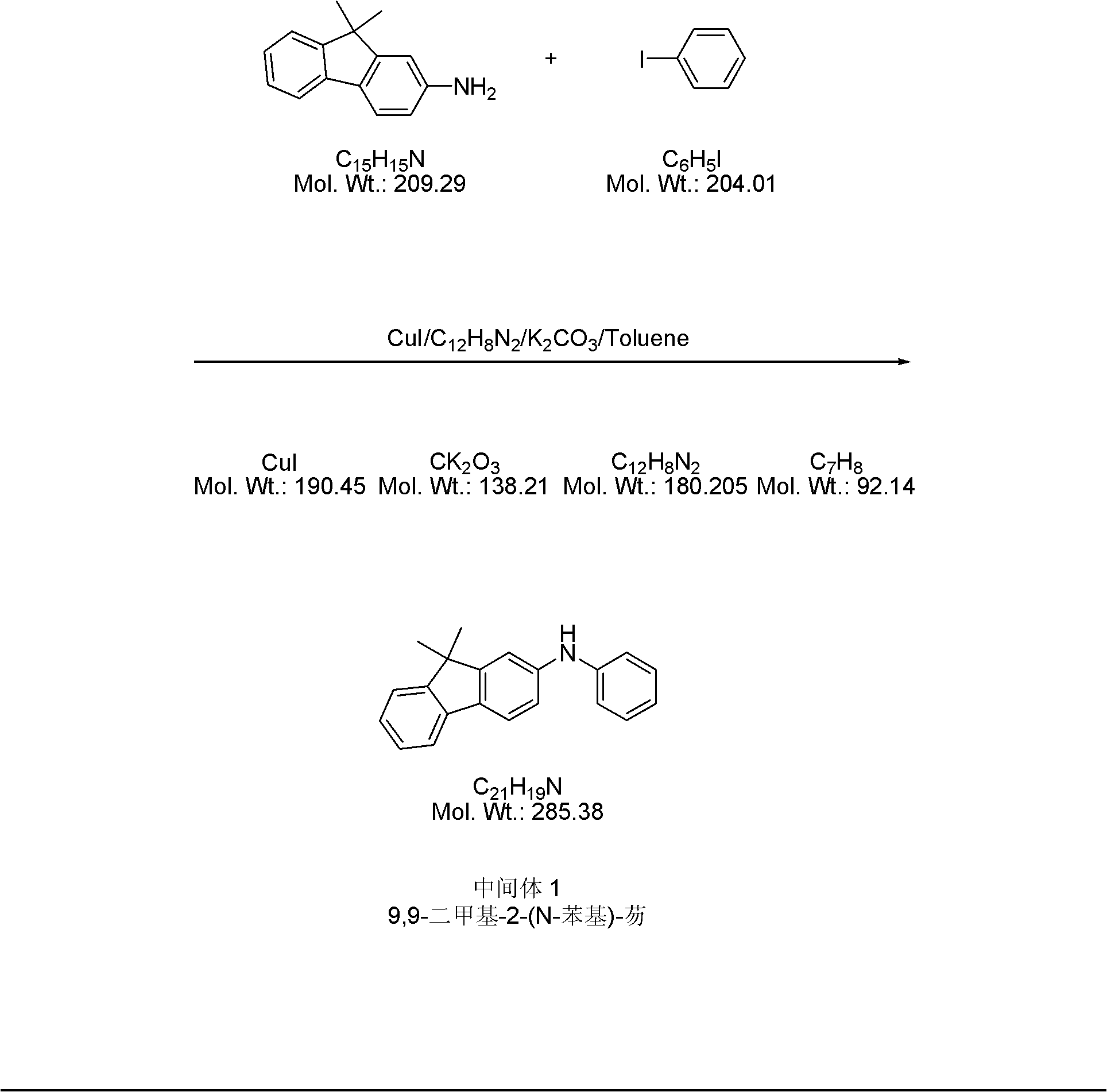
[0028] 1) Add 500 grams of 9,9-dimethyl-2-aminofluorene to a 5-liter four-necked bottle, and then add 487.56 grams of iodobenzene, 1500 grams of toluene, 4.56 grams of cuprous iodide, and 4.32 grams of phenanthrene phylloline and 500 grams of potassium carbonate. The above reaction solution was stirred under reflux for 6 hours, cooled to room temperature, filtered, and the filtrate was washed with water until the pH value of the aqueous phase was 7-8. The filtrate organic phase after washing is separated from the aqueous phase, the aqueous phase is discarded, and the organic phase is retained, and then the above-mentioned organic phase is concentrated to dryness using a rotary evaporator to obtain a crude product, and finally the crude product is subjected to silica gel column chromatography (the weight ratio of the crude product to silica gel After recrystallization and purification of 10) and ethyl acetate petroleum ether, 388 g of intermediate 1 (9,9-dimethyl-2-(N-phenyl)-f...
Embodiment 2
[0035] 1) Add 83 grams of 9,9-dimethyl-2-aminofluorene to a four-necked flask, then add 50 grams of iodobenzene, 300 grams of toluene, 0.92 grams of cuprous iodide, 0.87 grams of phenanthroline and carbonic acid Potassium 120 grams. The reaction solution was stirred under reflux for 6 hours, cooled to room temperature, filtered, and the filtrate was washed with water until the pH value of the aqueous phase was 7-8. The filtrate organic phase after washing is separated from the aqueous phase, the aqueous phase is discarded, and the organic phase is retained, and then the above-mentioned organic phase is concentrated to dryness using a rotary evaporator to obtain a crude product, and finally the crude product is subjected to silica gel column chromatography (the weight ratio of the crude product to silica gel 8) was purified by recrystallization from ethyl acetate petroleum ether to obtain 46 g of intermediate 1 (9,9-dimethyl-2-(N-phenyl)-fluorene), with a yield of 65%.
[0036...
Embodiment 3
[0042] 1) Add 70 grams of 9,9-dimethyl-2-aminofluorene to a four-necked flask, then add 35 grams of iodobenzene, 350 grams of toluene, 0.77 grams of cuprous iodide, 0.73 grams of phenanthroline and 130 grams of potassium carbonate. The reaction solution was stirred under reflux for 6 hours. Cool to room temperature, filter, and wash the filtrate with water until the pH of the aqueous phase is 7-8. The filtrate organic phase after washing is separated from the aqueous phase, the aqueous phase is discarded, and the organic phase is retained, and then the above-mentioned organic phase is concentrated to dryness using a rotary evaporator to obtain a crude product, and finally the crude product is subjected to silica gel column chromatography (the weight ratio of the crude product to silica gel 36 g of intermediate 1 (9,9-dimethyl-2-(N-phenyl)-fluorene) was obtained after recrystallization and purification of 6) and ethyl acetate petroleum ether, with a yield of 73%.
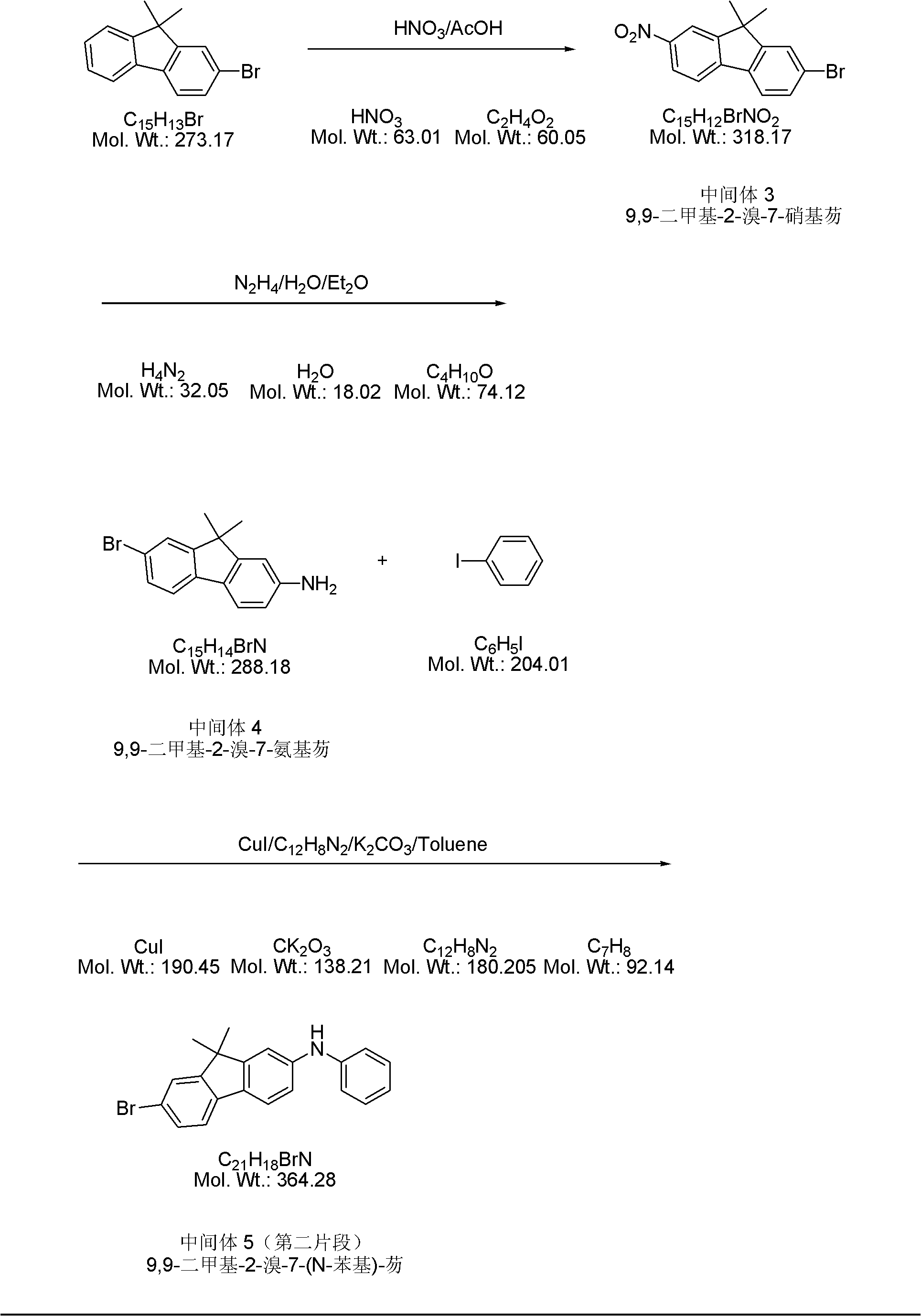
[0043] 2) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com