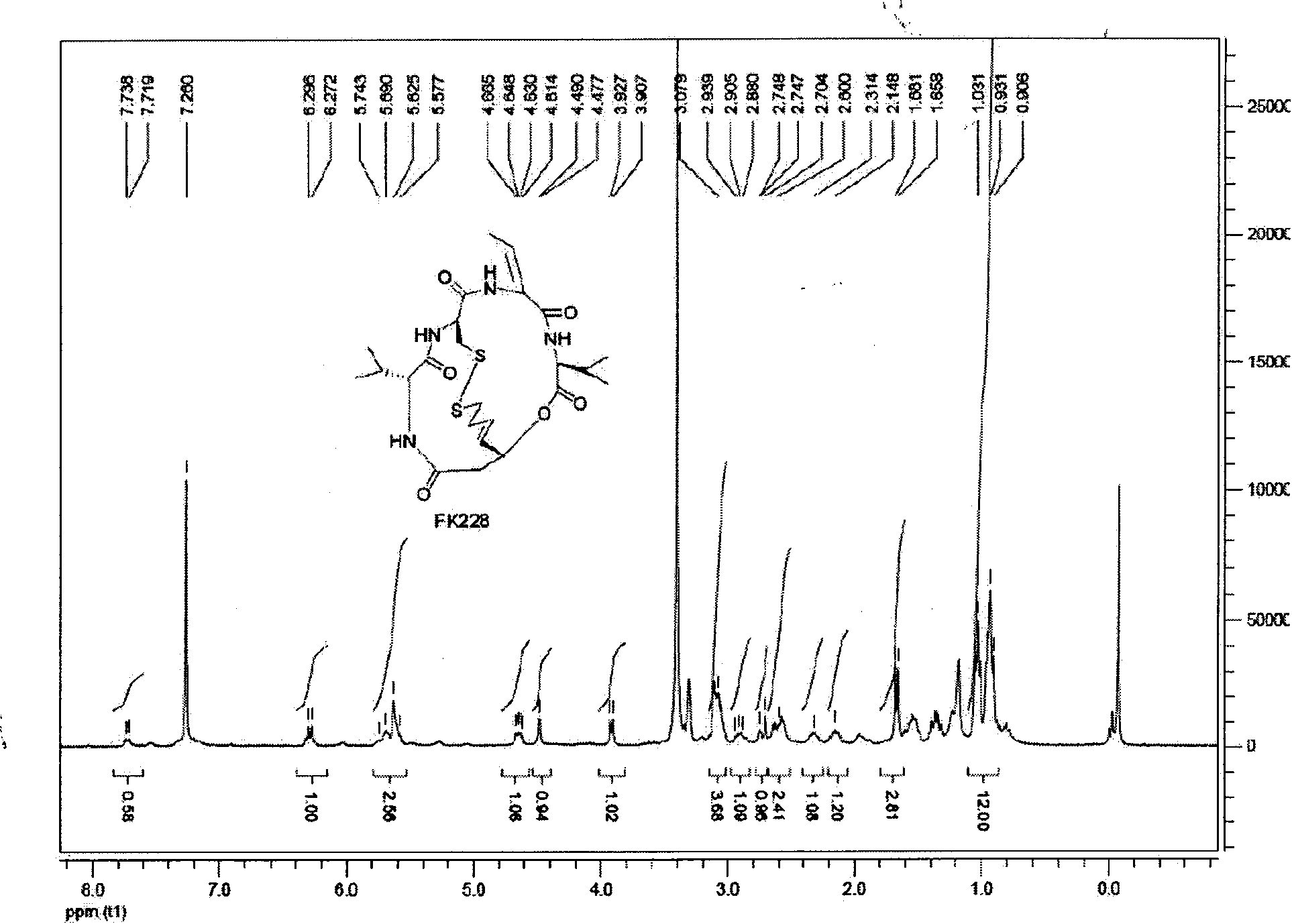
Preparation method of histone deacetylase inhibitor FK228
A technology of FK228 and condensing agent, which is applied in the direction of chemical instruments and methods, cyclic peptides, etc., can solve the problems of limiting the preparation of FK228, and achieve the effects of avoiding product deterioration, high yield, and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of compound (II)
[0029] At room temperature, 30.5g of compound (I) was dissolved in 500mL of dichloromethane and 50mL of piperidine was added, stirred for 30 minutes, and the above solution was slowly added dropwise to 4L of vigorously stirred 14.4gHATU and 10mL of diisopropylethylamine. Dichloromethane solution, added dropwise for more than 3 hours, stirred for 12 hours, quenched with saturated ammonium chloride, extracted three times with dichloromethane, washed the organic layer with saturated brine, dried, concentrated, and separated by column chromatography (ethyl acetate:petroleum ether) =1:4-2:1) to obtain 12.2 g of white solid.
[0030] Synthesis of compound (III) namely FK228
[0031] At room temperature, 24.8g iodine simple substance is dissolved in 20L methanol / dichloromethane ratio is 1 / 10 in the mixed solvent, under vigorous stirring slowly drips 10L methanol / dichloromethane ratio of 11.5g compound (I) is 1 / 10 solution, dropwise for not less t...
Embodiment 2
[0034] Synthesis of compound (II)
[0035] At room temperature, 30.5g of compound (I) was dissolved in 500mL of dichloromethane and 50mL of triethylamine was added, stirred for 12 hours, and the above solution was slowly added dropwise to 4L of vigorously stirred 28.8gHATU and 10mL of diisopropylethylamine. in dichloromethane solution, added dropwise for more than 3 hours, stirred for 12 hours, quenched with saturated ammonium chloride, extracted three times with dichloromethane, washed the organic layer with saturated brine, dried, concentrated, and separated by column chromatography (ethyl acetate: petroleum ether=1:4-2:1) to obtain 13.0 g of white solid.
[0036] Synthesis of compound (III) namely FK228
[0037] At room temperature, 24.8g iodine simple substance is dissolved in 20L methanol / dichloromethane ratio is 6 / 4 in the mixed solvent, slowly drips 11.5g compound (I) under vigorous stirring 10L methanol / dichloromethane ratio is 6 / 4 solution, dropwise for not less tha...
Embodiment 3
[0040] Synthesis of compound (II)
[0041] At room temperature, 30.5g of compound (I) was dissolved in 500mL of dichloromethane and 50mL of morpholine was added, stirred for 3 hours, and the above solution was slowly added dropwise to 4L of vigorously stirred 14.4gHATU and 5mL of diisopropylethylamine. Dichloromethane solution, added dropwise for more than 4 hours, stirred for 12 hours, quenched with saturated ammonium chloride, extracted three times with dichloromethane, washed the organic layer with saturated brine, dried, concentrated, and separated by column chromatography (ethyl acetate:petroleum ether) =1:4-2:1) to obtain 14.9 g of white solid.
[0042] Synthesis of compound (III) namely FK228
[0043] At room temperature, 12.4g elemental iodine was dissolved in 10L methanol / dichloromethane ratio of 9 / 1 mixed solvent, and slowly added dropwise 11.5g of compound (I) in 5L methanol / dichloromethane ratio of 9 / 1 under vigorous stirring Solution, dropwise for not less than ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com