I-type canine adenovirus attenuated vaccine strain and application thereof
A technology for canine adenovirus and attenuated vaccines, which can be applied to antiviral agents, inactivation/attenuation, nervous system diseases, etc. It can solve the problems of high cost and low antigen specificity, and achieve good protection and immune protection effect, good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1I
[0014] Isolation and identification of embodiment 1 type I canine adenovirus (CAV-H) isolate
[0015] 1 Materials and methods
[0016] 1.1 Disease material: The affected dog came from Heilongjiang Province. The clinical manifestations were elevated body temperature, watery eyes and nose, and bloody stool. The sick dog died within 3 days of the illness. Intestinal contents were collected for virus isolation.
[0017] 1.2 Cells: MDCK (generation 74-94, the same below) cell line was purchased from China Veterinary Drug Control Institute.
[0018] 1.3 Experimental animals: 2-4 month-old puppies (CAV antibody titer ≤ 1:2) were selected and provided by the Department of Experimental Zoology of Harbin Medical University. After deworming and 1w observation, it was determined that the healthy ones were available.
[0019] 1.4 Serum: canine anti-CAV (SN≥1:16) standard positive serum and negative serum were made by the inventor's laboratory.
[0020] 1.5 Isolation of virus: Take the i...
Embodiment 2I
[0079] The cultivation of embodiment 2 type I canine adenovirus attenuated vaccine strain CAV-HR
[0080] 1 Materials and methods
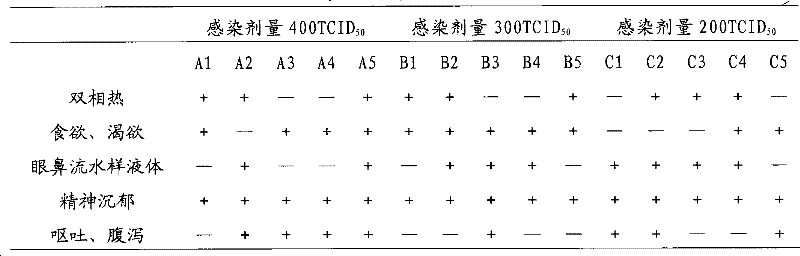
[0081] 1.1 Test materials The test animals are healthy dogs aged 2 to 4 months (CAV antibody titer ≤ 1:2); anti-canine adenovirus-specific serum and 1% red blood cell suspension are prepared by our laboratory, and the strong toxicity is CAV-H 6 generation.
[0082] 1.2 Cultivation and identification of CAV-H attenuated strain
[0083] 1.2.1 Cultivation of attenuated CAV-H strain
[0084] CAV-H was passaged through MDCK cells to passage 110, during which clonal purification was carried out 3 times.
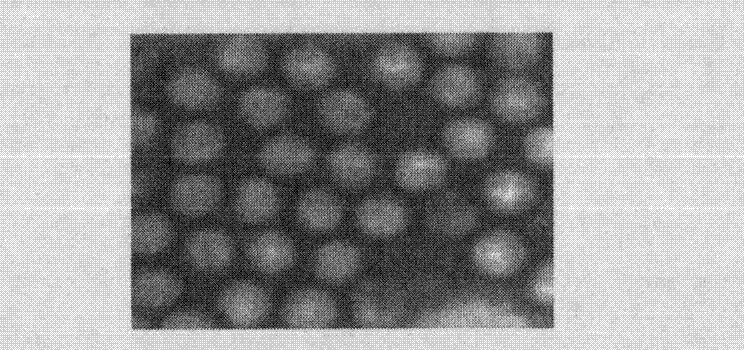
[0085] 1.2.2 Morphological observation of attenuated CAV-H strain
[0086] Centrifuge the harvested CAV-H 5, 10, 20, 30, 50, 70, 90, and 110 passage virus cell cultures at 5000r / min for 30min, take the supernatant and ultracentrifuge at 20000r / min, centrifuge at 4°C for 2h, and use for precipitation Appropriate amount of PBS (pH 7.0) suspended, ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com